Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry
- PMID: 16940508
- PMCID: PMC1563945
- DOI: 10.1128/JVI.00843-06
Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry
Abstract
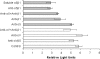
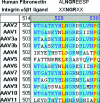
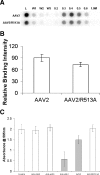
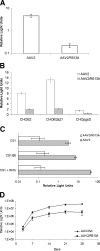
Integrins have been implicated as coreceptors in the infectious pathways of several nonenveloped viruses. For example, adenoviruses are known to interact with alphaV integrins by virtue of a high-affinity arginine-glycine-aspartate (RGD) domain present in the penton bases of the capsids. In the case of adeno-associated virus type 2 (AAV2), which lacks this RGD motif, integrin alphaVbeta5 has been identified as a coreceptor for cellular entry. However, the molecular determinants of AAV2 capsid-integrin interactions and the potential exploitation of alternative integrins as coreceptors by AAV2 have not been established thus far. In this report, we demonstrate that integrin alpha5beta1 serves as an alternative coreceptor for AAV2 infection in human embryonic kidney 293 cells. Such interactions appear to be mediated by a highly conserved domain that contains an asparagine-glycine-arginine (NGR) motif known to bind alpha5beta1 integrin with moderate affinity. The mutation of this domain reduces transduction efficiency by an order of magnitude relative to that of wild-type AAV2 vectors in vitro and in vivo. Further characterization of mutant and wild-type AAV2 capsids through transduction assays in cell lines lacking specific integrins, cell adhesion studies, and cell surface/solid-phase binding assays confirmed the role of the NGR domain in promoting AAV2-integrin interactions. Molecular modeling studies suggest that NGR residues form a surface loop close to the threefold axis of symmetry adjacent to residues previously implicated in binding heparan sulfate, the primary receptor for AAV2. The aforementioned results suggest that the internalization of AAV2 in 293 cells might follow a "click-to-fit" mechanism that involves the cooperative binding of heparan sulfate and alpha5beta1 integrin by the AAV2 capsids.
Figures



References
-
- Berns, K. I., and C. Giraud. 1996. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 218:1-23. - PubMed
-
- Di Pasquale, G., B. L. Davidson, C. S. Stein, I. Martins, D. Scudiero, A. Monks, and J. A. Chiorini. 2003. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 9:1306-1312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

