Integrin-mediated adhesion regulates membrane order
- PMID: 16943184
- PMCID: PMC2064315
- DOI: 10.1083/jcb.200603034
Integrin-mediated adhesion regulates membrane order
Abstract
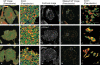
The properties of cholesterol-dependent domains (lipid rafts) in cell membranes have been controversial. Because integrin-mediated cell adhesion and caveolin both regulate trafficking of raft components, we investigated the effects of adhesion and caveolin on membrane order. The fluorescent probe Laurdan and two-photon microscopy revealed that focal adhesions are highly ordered; in fact, they are more ordered than caveolae or domains that stain with cholera toxin subunit B (CtxB). Membrane order at focal adhesion depends partly on phosphorylation of caveolin1 at Tyr14, which localizes to focal adhesions. Detachment of cells from the substratum triggers a rapid, caveolin-independent decrease in membrane order, followed by a slower, caveolin-dependent decrease that correlates with internalization of CtxB-stained domains. Endocytosed CtxB domains also become more fluid. Thus, membrane order is highly dependent on caveolae and focal adhesions. These results show that lipid raft properties are conferred by assembly of specific protein complexes. The ordered state within focal adhesions may have important consequences for signaling at these sites.
Figures


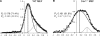


References
-
- Bagatolli, L.A., S.A. Sanchez, T. Hazlett, and E. Gratton. 2003. Giant vesicles, Laurdan, and two-photon fluorescence microscopy: evidence of lipid lateral separation in bilayers. Methods Enzymol. 360:481–500. - PubMed
-
- Brown, D.A., and E. London. 1998. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164:103–114. - PubMed
-
- Chapman, H.A., Y. Wei, D.I. Simon, and D.A. Waltz. 1999. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb. Haemost. 82:291–297. - PubMed
-
- del Pozo, M.A., W.B. Kiosses, N.B. Alderson, N. Meller, K.M. Hahn, and M.A. Schwartz. 2002. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 4:232–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

