Novel mechanism of resistance to glycopeptide antibiotics in Enterococcus faecium
- PMID: 16943188
- PMCID: PMC2084264
- DOI: 10.1074/jbc.M606920200
Novel mechanism of resistance to glycopeptide antibiotics in Enterococcus faecium
Abstract
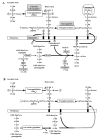
Glycopeptides and beta-lactams are the major antibiotics available for the treatment of infections due to Gram-positive bacteria. Emergence of cross-resistance to these drugs by a single mechanism has been considered as unlikely because they inhibit peptidoglycan polymerization by different mechanisms. The glycopeptides bind to the peptidyl-D-Ala(4)-D-Ala(5) extremity of peptidoglycan precursors and block by steric hindrance the essential glycosyltransferase and D,D-transpeptidase activities of the penicillin-binding proteins (PBPs). The beta-lactams are structural analogues of D-Ala(4)-D-Ala(5) and act as suicide substrates of the D,D-transpeptidase module of the PBPs. Here we have shown that bypass of the PBPs by the recently described beta-lactam-insensitive L,D-transpeptidase from Enterococcus faecium (Ldt(fm)) can lead to high level resistance to glycopeptides and beta-lactams. Cross-resistance was selected by glycopeptides alone or serially by beta-lactams and glycopeptides. In the corresponding mutants, UDP-MurNAc-pentapeptide was extensively converted to UDP-MurNAc-tetrapeptide following hydrolysis of D-Ala(5), thereby providing the substrate of Ldt(fm). Complete elimination of D-Ala(5), a residue essential for glycopeptide binding, was possible because Ldt(fm) uses the energy of the L-Lys(3)-D-Ala(4) peptide bond for cross-link formation in contrast to PBPs, which use the energy of the D-Ala(4)-D-Ala(5) bond. This novel mechanism of glycopeptide resistance was unrelated to the previously identified replacement of D-Ala(5) by D-Ser or D-lactate.
Figures




Similar articles
-
Activation of the L,D-transpeptidation peptidoglycan cross-linking pathway by a metallo-D,D-carboxypeptidase in Enterococcus faecium.Mol Microbiol. 2010 Feb;75(4):874-85. doi: 10.1111/j.1365-2958.2009.07014.x. Epub 2009 Dec 16. Mol Microbiol. 2010. PMID: 20025663
-
Balance between two transpeptidation mechanisms determines the expression of beta-lactam resistance in Enterococcus faecium.J Biol Chem. 2002 Sep 27;277(39):35801-7. doi: 10.1074/jbc.M204319200. Epub 2002 Jun 19. J Biol Chem. 2002. PMID: 12077139
-
Unexpected inhibition of peptidoglycan LD-transpeptidase from Enterococcus faecium by the beta-lactam imipenem.J Biol Chem. 2007 Oct 19;282(42):30414-22. doi: 10.1074/jbc.M704286200. Epub 2007 Jul 23. J Biol Chem. 2007. PMID: 17646161
-
Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria.FEMS Microbiol Rev. 2008 Mar;32(2):386-408. doi: 10.1111/j.1574-6976.2007.00097.x. Epub 2008 Feb 11. FEMS Microbiol Rev. 2008. PMID: 18266857 Review.
-
Penicillin-binding proteins and peptidoglycan peptide-interacting proteins.Microbiol Sci. 1984 Dec;1(9):211-4. Microbiol Sci. 1984. PMID: 6444131 Review.
Cited by
-
Mechanisms of antibiotic resistance in enterococci.Expert Rev Anti Infect Ther. 2014 Oct;12(10):1221-36. doi: 10.1586/14787210.2014.956092. Expert Rev Anti Infect Ther. 2014. PMID: 25199988 Free PMC article. Review.
-
The Characterization of Enterococcus Genus: Resistance Mechanisms and Inflammatory Bowel Disease.Open Med (Wars). 2020 Apr 3;15:211-224. doi: 10.1515/med-2020-0032. eCollection 2020. Open Med (Wars). 2020. PMID: 32292819 Free PMC article.
-
Mutation landscape of acquired cross-resistance to glycopeptide and β-lactam antibiotics in Enterococcus faecium.Antimicrob Agents Chemother. 2015 Sep;59(9):5306-15. doi: 10.1128/AAC.00634-15. Epub 2015 Jun 15. Antimicrob Agents Chemother. 2015. PMID: 26077262 Free PMC article.
-
Structural variations of the cell wall precursor lipid II and their influence on binding and activity of the lipoglycopeptide antibiotic oritavancin.Antimicrob Agents Chemother. 2015 Feb;59(2):772-81. doi: 10.1128/AAC.02663-14. Epub 2014 Nov 17. Antimicrob Agents Chemother. 2015. PMID: 25403671 Free PMC article.
-
β-Lactam Resistance Mechanisms: Gram-Positive Bacteria and Mycobacterium tuberculosis.Cold Spring Harb Perspect Med. 2016 May 2;6(5):a025221. doi: 10.1101/cshperspect.a025221. Cold Spring Harb Perspect Med. 2016. PMID: 27091943 Free PMC article. Review.
References
-
- Walsh C. Nature. 2000;406:775–781. - PubMed
-
- Rammelkamp CH, Maxon T. Proc Soc Exper Biol Med. 1942;51:386–389.
-
- Abraham EP, Chain E. Nature. 1940;146:837.
-
- Leclercq R, Derlot E, Duval J, Courvalin P. N Engl J Med. 1988;319:157–161. - PubMed
-
- Reynolds PE. Eur J Clin Microbiol Infect Dis. 1989;8:943–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

