Rotavirus infection is not associated with small intestinal fluid secretion in the adult mouse
- PMID: 16943290
- PMCID: PMC1642176
- DOI: 10.1128/JVI.00152-06
Rotavirus infection is not associated with small intestinal fluid secretion in the adult mouse
Abstract
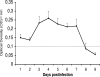
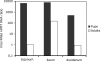
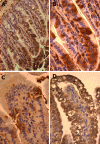
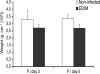
In contrast to humans, adult but not infant small animals are resistant to rotavirus diarrhea. The pathophysiological mechanism behind this age-restricted diarrhea is currently unresolved, and this question was investigated by studying the secretory state of the small intestines of adult mice infected with rotavirus. Immunohistochemistry and histological examinations revealed that rotavirus (strain EDIM) infects all parts of the small intestines of adult mice, with significant numbers of infected cells in the ilea at 2 and 4 days postinfection. Furthermore, quantitative PCR revealed that 100-fold more viral RNA was produced in the ilea than in the jejuna or duodena of adult mice. In vitro perfusion experiments of the small intestine did not reveal any significant changes in net fluid secretion among mice infected for 3 days or 4 days or in those that were noninfected (37 +/- 9 microl . h(-1) . cm(-1), 22 +/- 13 microl . h(-1) . cm(-1), and 33 +/- 6 microl . h(-1) . cm(-1), respectively) or in transmucosal potential difference (4.0 +/- 0.3 mV versus 3.9 +/- 0.4 mV), a marker for active chloride secretion, between control and rotavirus-infected mice. In vivo experiments also did not show any differences in potential difference between uninfected and infected small intestines. Furthermore, no significant differences in weight between infected and uninfected small intestines were found, nor were any differences in fecal output observed between infected and control mice. Altogether, these data suggest that rotavirus infection is not sufficient to stimulate chloride and water secretion from the small intestines of adult mice.
Figures

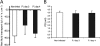

References
-
- Burns, J. W., A. A. Krishnaney, P. T. Vo, R. V. Rouse, L. J. Anderson, and H. B. Greenberg. 1995. Analyses of homologous rotavirus infection in the mouse model. Virology 207:143-153. - PubMed
-
- Ciarlet, M., M. A. Gilger, C. Barone, M. McArthur, M. K. Estes, and M. E. Conner. 1998. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology 251:343-360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

