Antagonistic regulation of beta-globin gene expression by helix-loop-helix proteins USF and TFII-I
- PMID: 16943425
- PMCID: PMC1592872
- DOI: 10.1128/MCB.01770-05
Antagonistic regulation of beta-globin gene expression by helix-loop-helix proteins USF and TFII-I
Abstract
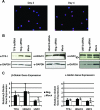
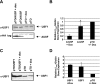
The human beta-globin genes are expressed in a developmental stage-specific manner in erythroid cells. Gene-proximal cis-regulatory DNA elements and interacting proteins restrict the expression of the genes to the embryonic, fetal, or adult stage of erythropoiesis. In addition, the relative order of the genes with respect to the locus control region contributes to the temporal regulation of the genes. We have previously shown that transcription factors TFII-I and USF interact with the beta-globin promoter in erythroid cells. Herein we demonstrate that reducing the activity of USF decreased beta-globin gene expression, while diminishing TFII-I activity increased beta-globin gene expression in erythroid cell lines. Furthermore, a reduction of USF activity resulted in a significant decrease in acetylated H3, RNA polymerase II, and cofactor recruitment to the locus control region and to the adult beta-globin gene. The data suggest that TFII-I and USF regulate chromatin structure accessibility and recruitment of transcription complexes in the beta-globin gene locus and play important roles in restricting beta-globin gene expression to the adult stage of erythropoiesis.
Figures






References
-
- Armstrong, J. A., J. J. Bieker, and B. M. Emerson. 1998. A SWI/SNF related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell 95:93-104. - PubMed
-
- Bender, M. A., J. N. Roach, J. Hallow, J. Close, R. Alami, E. E. Bouhassira, M. Groudine, and S. N. Fiering. 2001. Targeted deletion of 5′HS1 and 5′HS4 of the beta-globin locus control region reveals additive activity of the DNase I hypersensitive sites. Blood 98:2022-2027. - PubMed
-
- Bieker, J. J. 2001. Kruppel-like factors: three fingers in many pies. J. Biol. Chem. 276:34355-34358. - PubMed
-
- Bresnick, E. H., and G. Felsenfeld. 1993. Evidence that the transcription factor USF is a component of the human beta-globin locus control region heteromeric protein complex. J. Biol. Chem. 268:18824-18834. - PubMed
-
- Bulger, M., and M. Groudine. 1999. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 13:2465-2477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
