Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells
- PMID: 16943434
- PMCID: PMC1592869
- DOI: 10.1128/MCB.01040-06
Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells
Abstract
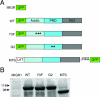
Syk tyrosine kinase and Src homology 2 (SH2) domain-containing leukocyte-specific phosphoprotein of 76 kDa (SLP-76) are signaling mediators activated downstream of immunoreceptor tyrosine-based activation motif (ITAM)-containing immunoreceptors and integrins. While the signaling cascades descending from integrins are similar to immunoreceptors, the mechanism of Syk activation and SLP-76 recruitment remains unclear. We used an in vivo structure-function approach to study the requirements for the domains of Syk and SLP-76 in immunoreceptor and integrin signaling. We found that both SH2 domains and the kinase domain of Syk are required for immunoreceptor-dependent signaling and cellular response via integrins. While the Gads-binding domain of SLP-76 is needed for immunoreceptor signaling, it appears dispensable for integrin signaling. Syk and SLP-76 also are required for initiating and/or maintaining separation between the blood and lymphatic vasculature. Therefore, we correlated the signaling requirement of the various domains of Syk and SLP-76 to their requirement in regulating vascular separation. Our data suggest ITAMs are required in Syk-dependent integrin signaling, demonstrate the separation of the structural features of SLP-76 to selectively support immunoreceptor versus integrin signaling, and provide evidence that the essential domains of SLP-76 for ITAM signals are those which most efficiently support separation between lymphatic and blood vessels.
Figures


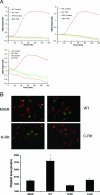
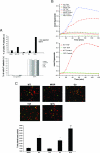
 , significant difference in spreading between MIGR or Y3F and WT or G2 (P < 0.001); #, significant difference in spreading between MTS and WT (P < 0.001).
, significant difference in spreading between MIGR or Y3F and WT or G2 (P < 0.001); #, significant difference in spreading between MTS and WT (P < 0.001).

References
-
- Abtahian, F., A. Guerriero, E. Sebzda, M. M. Lu, R. Zhou, A. Mocsai, E. E. Myers, B. Huang, D. G. Jackson, V. A. Ferrari, V. Tybulewicz, C. A. Lowell, J. J. Lepore, G. A. Koretzky, and M. L. Kahn. 2003. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299:247-251. - PMC - PubMed
-
- Boerth, N. J., J. J. Sadler, D. E. Bauer, J. L. Clements, S. M. Gheith, and G. A. Koretzky. 2000. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 192:1047-1058. - PMC - PubMed
-
- Bourette, R. P., S. Arnaud, G. M. Myles, J. P. Blanchet, L. R. Rohrschneider, and G. Mouchiroud. 1998. Mona, a novel hematopoietic-specific adaptor interacting with the macrophage colony-stimulating factor receptor, is implicated in monocyte/macrophage development. EMBO J. 17:7273-7281. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
