Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease
- PMID: 16943563
- PMCID: PMC6675335
- DOI: 10.1523/JNEUROSCI.2797-06.2006
Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease
Abstract
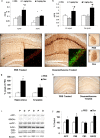
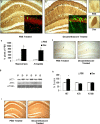
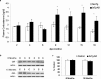
Various environmental and genetic factors influence the onset and progression of Alzheimer's disease (AD). Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which controls circulating levels of glucocorticoid hormones, occurs early in AD, resulting in increased cortisol levels. Disturbances of the HPA axis have been associated with memory impairments and may contribute to the cognitive decline that occurs in AD, although it is unknown whether such effects involve modulation of the amyloid beta-peptide (Abeta) and tau. Using in vitro and in vivo experiments, we report that stress-level glucocorticoid administration increases Abeta formation by increasing steady-state levels of amyloid precursor protein (APP) and beta-APP cleaving enzyme. Additionally, glucocorticoids augment tau accumulation, indicating that this hormone also accelerates the development of neurofibrillary tangles. These findings suggest that high levels of glucocorticoids, found in AD, are not merely a consequence of the disease process but rather play a central role in the development and progression of AD.
Figures


References
-
- Abraham I, Harkany T, Horvath KM, Veenema AH, Penke B, Nyakas C, Luiten PG. Chronic corticosterone administration dose-dependently modulates Abeta(1–42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J Neuroendocrinol. 2000;12:486–494. - PubMed
-
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, Weiner MF, Farlow MR, Sano M, Grundman M, Thal LJ. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology. 2000;54:588–593. - PubMed
-
- Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. - PubMed
-
- Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–S88. - PubMed
-
- Budas G, Coughlan CM, Seckl JR, Breen KC. The effect of corticosteroids on amyloid beta precursor protein/amyloid precursor-like protein expression and processing in vivo. Neurosci Lett. 1999;276:61–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
