Functional neural circuits for mental timekeeping
- PMID: 16944489
- PMCID: PMC6871423
- DOI: 10.1002/hbm.20285
Functional neural circuits for mental timekeeping
Abstract
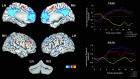
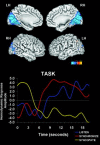
Theories of mental timekeeping suggest frontostriatal networks may mediate performance of tasks requiring precise timing. We assessed whether frontostriatal networks are functionally integrated during the performance of timing tasks. Functional magnetic resonance imaging (fMRI) data from 31 healthy adults were collected during performance of several different types of discrete interval timing tasks. Independent component analysis (ICA) was used to examine functional connectivity within frontostriatal circuits. ICA identifies groups of spatially discrete brain regions sharing similar patterns of hemodynamic signal change over time. The results confirm the existence of a frontostriatal neural timing circuit that includes anterior cingulate gyrus, supplementary motor area, bilateral anterior insula, bilateral putamen/globus pallidus, bilateral thalamus, and right superior temporal gyrus and supramarginal gyrus. Several other distinct neural circuits were identified that may represent the neurobiological substrates of different information processing stages of mental timekeeping. Small areas of right cerebellum were engaged in several of these circuits, suggesting that cerebellar function may be important in, but not the primary substrate of, the mental timing tasks used in this experiment. These findings are discussed within the context of current biological and information processing models of neural timekeeping.
Figures


References
-
- Assad JA (2003): Neural coding of behavioral relevance in parietal cortex. Curr Opin Neurobiol 13:194–197. - PubMed
-
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Vecchio F, Cola B, Rossi S, Miniussi C, Rossini PM (2004): Functional frontoparietal connectivity during short‐term memory as revealed by high‐resolution EEG coherence analysis. Behav Neurosci 118:687–697. - PubMed
-
- Behrmann M, Geng JJ, Shomstein S (2004): Parietal cortex and attention. Curr Opin Neurobiol 14:212–217. - PubMed
-
- Bell AJ, Sejnowski TJ (1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7:1129–1159. - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

