Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments
- PMID: 16945900
- PMCID: PMC1557634
- DOI: 10.1073/pnas.0606321103
Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments
Abstract
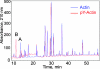
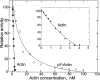
Dictyostelium actin was shown to become phosphorylated on Tyr-53 late in the developmental cycle and when cells in the amoeboid stage are subjected to stress but the phosphorylated actin had not been purified and characterized. We have separated phosphorylated and unphosphorylated actin and shown that Tyr-53 phosphorylation substantially reduces actin's ability to inactivate DNase I, increases actin's critical concentration, and greatly reduces its rate of polymerization. Tyr-53 phosphorylation substantially, if not completely, inhibits nucleation and elongation from the pointed end of actin filaments and reduces the rate of elongation from the barbed end. Negatively stained electron microscopic images of polymerized Tyr-53-phosphorylated actin show a variable mixture of small oligomers and filaments, which are converted to more typical, long filaments upon addition of myosin subfragment 1. Tyr-53-phosphorylated and unphosphorylated actin copolymerize in vitro, and phosphorylated and unphosphorylated actin colocalize in amoebae. Tyr-53 phosphorylation does not affect the ability of filamentous actin to activate myosin ATPase.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
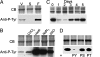






Similar articles
-
Modulation of actin structure and function by phosphorylation of Tyr-53 and profilin binding.Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11748-53. doi: 10.1073/pnas.0805852105. Epub 2008 Aug 8. Proc Natl Acad Sci U S A. 2008. PMID: 18689676 Free PMC article.
-
High levels of actin tyrosine phosphorylation: correlation with the dormant state of Dictyostelium spores.J Cell Sci. 1998 Oct;111 ( Pt 19):2923-32. doi: 10.1242/jcs.111.19.2923. J Cell Sci. 1998. PMID: 9730984
-
ForC lacks canonical formin activity but bundles actin filaments and is required for multicellular development of Dictyostelium cells.Eur J Cell Biol. 2013 Jun-Jul;92(6-7):201-12. doi: 10.1016/j.ejcb.2013.07.001. Epub 2013 Jul 11. Eur J Cell Biol. 2013. PMID: 23906540
-
Toward the structure of dynamic membrane-anchored actin networks: an approach using cryo-electron tomography.Cell Adh Migr. 2007 Jul-Sep;1(3):145-8. doi: 10.4161/cam.1.3.4662. Epub 2007 Jul 5. Cell Adh Migr. 2007. PMID: 19262130 Free PMC article. Review.
-
Microfilament dynamics: regulation of actin polymerization by actin-fragmin kinase and phosphatases.Adv Enzyme Regul. 1995;35:199-227. doi: 10.1016/0065-2571(94)00013-s. Adv Enzyme Regul. 1995. PMID: 7572344 Review.
Cited by
-
Towards a molecular understanding of the apicomplexan actin motor: on a road to novel targets for malaria remedies?Acta Crystallogr F Struct Biol Commun. 2015 May;71(Pt 5):500-13. doi: 10.1107/S2053230X1500391X. Epub 2015 Apr 16. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 25945702 Free PMC article. Review.
-
Expression of Y53A-actin in Dictyostelium disrupts the cytoskeleton and inhibits intracellular and intercellular chemotactic signaling.J Biol Chem. 2010 Sep 3;285(36):27713-25. doi: 10.1074/jbc.M110.116277. Epub 2010 Jul 7. J Biol Chem. 2010. PMID: 20610381 Free PMC article.
-
Omics Analyses of Trichomonas vaginalis Actin and Tubulin and Their Participation in Intercellular Interactions and Cytokinesis.Genes (Basel). 2022 Jun 15;13(6):1067. doi: 10.3390/genes13061067. Genes (Basel). 2022. PMID: 35741829 Free PMC article.
-
Lysine acetylation of cytoskeletal proteins: Emergence of an actin code.J Cell Biol. 2020 Dec 7;219(12):e202006151. doi: 10.1083/jcb.202006151. J Cell Biol. 2020. PMID: 33044556 Free PMC article. Review.
-
Age-Onset Phosphorylation of a Minor Actin Variant Promotes Intestinal Barrier Dysfunction.Dev Cell. 2019 Dec 2;51(5):587-601.e7. doi: 10.1016/j.devcel.2019.11.001. Dev Cell. 2019. PMID: 31794717 Free PMC article.
References
-
- Loomis WF. Dictyostelium discoideum: A Developmental System. New York: Academic; 1975.
-
- Gauthier ML, Lydan MA, O’Day DH, Cotter DA. Cell Signal. 1997;9:79–83. - PubMed
-
- Kishi Y, Clements C, Mahadeo DC, Cotter DA, Sameshima M. J Cell Sci. 1998;111:2923–2932. - PubMed
-
- Sameshima M, Kishi Y, Osumi M, Minamikawa-Tachino R, Mahadeo D, Cotter DA. J Struct Biol. 2001;136:7–19. - PubMed
-
- Schweiger A, Mihalache O, Ecke M, Gerisch G. J Cell Sci. 1992;102:601–609. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

