The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms
- PMID: 16945909
- PMCID: PMC1564243
- DOI: 10.1073/pnas.0605928103
The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms
Abstract
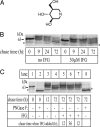
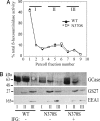
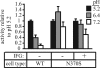
Gaucher disease is a lysosomal storage disorder caused by deficiency in lysosomal acid beta-glucosidase (GlcCerase), the enzyme responsible for the catabolism of glucosylceramide. One of the most prevalent disease-causing mutations, N370S, results in an enzyme with lower catalytic activity and impaired exit from the endoplasmic reticulum. Here, we report that the iminosugar isofagomine (IFG), an active-site inhibitor, increases GlcCerase activity 3.0 +/- 0.6-fold in N370S fibroblasts by several mechanisms. A major effect of IFG is to facilitate the folding and transport of newly synthesized GlcCerase in the endoplasmic reticulum, thereby increasing the lysosomal pool of the enzyme. In addition, N370S GlcCerase synthesized in the presence of IFG exhibits a shift in pH optimum from 6.4 to 5.2 and altered sensitivity to SDS. Although IFG fully inhibits GlcCerase in the lysosome in an in situ assay, washout of the drug leads to partial recovery of GlcCerase activity within 4 h and full recovery by 24 h. These findings provide support for the possible use of active-site inhibitors in the treatment of some forms of Gaucher disease.
Conflict of interest statement
Conflict of interest statement: S.A.K. is a member of the Scientific Advisory Board of Amicus Therapeutics.
Figures
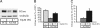
Similar articles
-
Selective action of the iminosugar isofagomine, a pharmacological chaperone for mutant forms of acid-beta-glucosidase.Biochem Pharmacol. 2007 May 1;73(9):1376-83. doi: 10.1016/j.bcp.2006.12.015. Epub 2006 Dec 15. Biochem Pharmacol. 2007. PMID: 17217920 Free PMC article.
-
Hydrophilic iminosugar active-site-specific chaperones increase residual glucocerebrosidase activity in fibroblasts from Gaucher patients.FEBS J. 2006 Sep;273(17):4082-92. doi: 10.1111/j.1742-4658.2006.05410.x. FEBS J. 2006. PMID: 16934036
-
Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease.Nat Chem Biol. 2007 Feb;3(2):101-7. doi: 10.1038/nchembio850. Epub 2006 Dec 24. Nat Chem Biol. 2007. PMID: 17187079
-
Acid beta-glucosidase: insights from structural analysis and relevance to Gaucher disease therapy.Biol Chem. 2008 Nov;389(11):1361-9. doi: 10.1515/BC.2008.163. Biol Chem. 2008. PMID: 18783340 Review.
-
Glucocerebrosidase inhibitors for the treatment of Gaucher disease.Future Med Chem. 2013 Apr;5(5):573-90. doi: 10.4155/fmc.13.14. Future Med Chem. 2013. PMID: 23573974 Review.
Cited by
-
X-ray and biochemical analysis of N370S mutant human acid β-glucosidase.J Biol Chem. 2011 Jan 7;286(1):299-308. doi: 10.1074/jbc.M110.150433. Epub 2010 Oct 27. J Biol Chem. 2011. PMID: 20980263 Free PMC article.
-
Upregulation of Sortilin, a Lysosomal Sorting Receptor, Corresponds with Reduced Bioavailability of Latent TGFβ in Mucolipidosis II Cells.Biomolecules. 2020 Apr 26;10(5):670. doi: 10.3390/biom10050670. Biomolecules. 2020. PMID: 32357547 Free PMC article.
-
Neuronopathic Gaucher disease: dysregulated mRNAs and miRNAs in brain pathogenesis and effects of pharmacologic chaperone treatment in a mouse model.Hum Mol Genet. 2015 Dec 15;24(24):7031-48. doi: 10.1093/hmg/ddv404. Epub 2015 Sep 29. Hum Mol Genet. 2015. PMID: 26420838 Free PMC article.
-
Parkinson's Disease Associated with GBA Gene Mutations: Molecular Aspects and Potential Treatment Approaches.Acta Naturae. 2021 Apr-Jun;13(2):70-78. doi: 10.32607/actanaturae.11031. Acta Naturae. 2021. PMID: 34377557 Free PMC article.
-
Allosteric Modulation of GCase Enhances Lysosomal Activity and Reduces ER Stress in GCase-Related Disorders.Int J Mol Sci. 2025 May 6;26(9):4392. doi: 10.3390/ijms26094392. Int J Mol Sci. 2025. PMID: 40362629 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

