The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms
- PMID: 16945909
- PMCID: PMC1564243
- DOI: 10.1073/pnas.0605928103
The iminosugar isofagomine increases the activity of N370S mutant acid beta-glucosidase in Gaucher fibroblasts by several mechanisms
Abstract
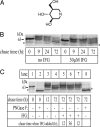
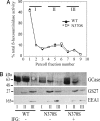
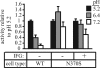
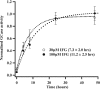
Gaucher disease is a lysosomal storage disorder caused by deficiency in lysosomal acid beta-glucosidase (GlcCerase), the enzyme responsible for the catabolism of glucosylceramide. One of the most prevalent disease-causing mutations, N370S, results in an enzyme with lower catalytic activity and impaired exit from the endoplasmic reticulum. Here, we report that the iminosugar isofagomine (IFG), an active-site inhibitor, increases GlcCerase activity 3.0 +/- 0.6-fold in N370S fibroblasts by several mechanisms. A major effect of IFG is to facilitate the folding and transport of newly synthesized GlcCerase in the endoplasmic reticulum, thereby increasing the lysosomal pool of the enzyme. In addition, N370S GlcCerase synthesized in the presence of IFG exhibits a shift in pH optimum from 6.4 to 5.2 and altered sensitivity to SDS. Although IFG fully inhibits GlcCerase in the lysosome in an in situ assay, washout of the drug leads to partial recovery of GlcCerase activity within 4 h and full recovery by 24 h. These findings provide support for the possible use of active-site inhibitors in the treatment of some forms of Gaucher disease.
Conflict of interest statement
Conflict of interest statement: S.A.K. is a member of the Scientific Advisory Board of Amicus Therapeutics.
Figures
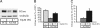
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

