Tobacco plastid ribosomal protein S18 is essential for cell survival
- PMID: 16945948
- PMCID: PMC1636375
- DOI: 10.1093/nar/gkl634
Tobacco plastid ribosomal protein S18 is essential for cell survival
Abstract
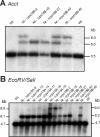
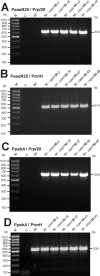
Plastid genomes contain a conserved set of genes most of which are involved in either photosynthesis or gene expression. Among the ribosomal protein genes present in higher plant plastid genomes, rps18 is special in that it is absent from the plastid genomes of several non-green unicellular organisms, including Euglena longa and Toxoplasma gondii. Here we have tested whether the ribosomal protein S18 is required for translation by deleting the rps18 gene from the tobacco plastid genome. We report that, while deletion of the rps18 gene was readily obtained, no homoplasmic Deltarps18 plants or leaf sectors could be isolated. Instead, segregation into homoplasmy led to severe defects in leaf development suggesting that the knockout of rps18 is lethal and the S18 protein is required for cell survival. Our data demonstrate that S18 is indispensable for plastid ribosome function in tobacco and support an essential role for plastid translation in plant development. Moreover, we demonstrate the occurrence of flip-flop recombination on short inverted repeat sequences which generates different isoforms of the transformed plastid genome that differ in the orientation a 70 kb segment in the large single-copy region. However, infrequent occurrence of flip-flop recombination and random segregation of plastid genomes result in the predominant presence of only one of the isoforms in many tissue samples. Implications for the interpretation of chloroplast transformation experiments and vector design are discussed.
Figures





References
-
- Mache R. Chloroplast ribosomal proteins and their genes. Plant Sci. 1990;72:1–12.
-
- Sugiura M., Hirose T., Sugita M. Evolution and mechanism of translation in chloroplasts. Annu. Rev. Genet. 1998;32:437–459. - PubMed
-
- Gockel G., Hachtel W., Baier S., Fliss C., Henke M. Genes for components of the chloroplast translational apparatus are conserved in the reduced 73-kb plastid DNA of the nonphotosynthetic euglenoid flagellate Astasia longa. Curr. Genet. 1994;26:256–262. - PubMed
-
- Wilson R.J.M., Denny P.W., Preiser P.R., Rangachari K., Roberts K., Roy A., Whyte A., Strath M., Moore D.J., Moore P.W., et al. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 1996;261:155–172. - PubMed

