CAG*CTG repeat instability in cultured human astrocytes
- PMID: 16945950
- PMCID: PMC1636369
- DOI: 10.1093/nar/gkl614
CAG*CTG repeat instability in cultured human astrocytes
Abstract
Cells of the central nervous system (CNS) are prone to the devastating consequences of trinucleotide repeat (TNR) expansion. Some CNS cells, including astrocytes, show substantial TNR instability in affected individuals. Since astrocyte enrichment occurs in brain regions sensitive to neurodegeneration and somatic TNR instability, immortalized SVG-A astrocytes were used as an ex vivo model to mimic TNR mutagenesis. Cultured astrocytes produced frequent (up to 2%) CAG.CTG contractions in a sequence-specific fashion, and an apparent threshold for instability was observed between 25 and 33 repeats. These results suggest that cultured astrocytes recapitulate key features of TNR mutagenesis. Furthermore, contractions were influenced by DNA replication through the repeat, suggesting that instability can arise by replication-based mechanisms in these cells. This is a crucial mechanistic point, since astrocytes in the CNS retain proliferative capacity throughout life and could be vulnerable to replication-mediated TNR instability. The presence of interruptions led to smaller but more frequent contractions, compared to a pure repeat, and the interruptions were sometimes deleted to form a perfect tract. In summary, we suggest that CAG.CTG repeat instability in cultured astrocytes is dynamic and replication-driven, suggesting that TNR mutagenesis may be influenced by the proliferative capacity of key CNS cells.
Figures
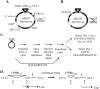
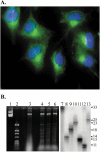

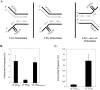
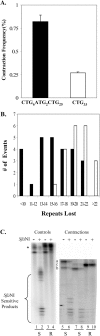
References
-
- Paulson H.L., Fischbeck K.H. Trinucleotide repeats in neurogenetic disorders. Annu. Rev. Neurosci. 1996;19:79–107. - PubMed
-
- Mirkin S.M. Molecular models for repeat expansions. Chemtracts-Biochem. Mol. Biol. 2004;17:639–662.
-
- Pearson C.E., Edamura K.N., Cleary J.D. Repeat instability: mechanisms of dynamic mutations. Nature Rev. Genet. 2005;6:729–742. - PubMed
-
- Kovtun I.V., McMurray C.T. Trinucleotide expansion in haploid germ cells by gap repair. Nature Genet. 2001;27:407–411. - PubMed
-
- Gomes-Pereira M., Fortune M.T., Monckton D.G. Mouse tissue culture models of unstable triplet repeats: in vitro selection for larger alleles, mutational expansion bias and tissue specificity, but no association with cell division rates. Hum. Mol. Genet. 2001;10:845–854. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

