Frequency-dependent selection of reflexes by pudendal afferents in the cat
- PMID: 16945977
- PMCID: PMC2000666
- DOI: 10.1113/jphysiol.2006.111815
Frequency-dependent selection of reflexes by pudendal afferents in the cat
Abstract
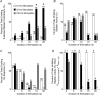
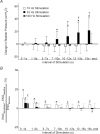
Activation of urethral or genital afferents of the pudendal nerve can elicit or inhibit micturition, and low frequency stimulation of the compound pudendal nerve (PN) is known to produce a continence response. The present study demonstrates that PN stimulation also can elicit a micturition-like response and that the response to PN stimulation is dependent on stimulation frequency. We measured the changes in bladder pressure and external urethral sphincter (EUS) electroneurogram (ENG) evoked by PN stimulation before and up to 16 h after spinal cord transection (SCT) in cats anaesthetized with alpha-chloralose. Low frequency (10 Hz) stimulation elicited a continence-like response, including inhibition of the bladder and activation of the EUS, but mid-frequency (33 Hz) stimulation produced a micturition-like response, including excitation of the bladder without activation of the EUS. The dependence of the response on stimulus frequency was linked to interpulse interval as the same number of pulses at 10, 33 and 100 Hz produced different responses. Stimulation of the PN at 33 Hz produced bladder contractions before and 8 h after SCT provided the bladder contained a minimum volume of fluid. Only mid-range frequency stimulation with sufficient stimulus train duration produced a reduction in EUS ENG activity before and after SCT. In addition to a continence-like response, PN stimulation can also elicit a micturition-like response, and this response is dependent on stimulation frequency, stimulus train duration, and bladder volume. The ability to control the two principal functions of the bladder by pudendal nerve stimulation is an exciting prospect for neurorehabilitation.
Figures
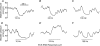

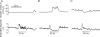
Similar articles
-
Spinal micturition reflex mediated by afferents in the deep perineal nerve.J Neurophysiol. 2005 May;93(5):2688-97. doi: 10.1152/jn.00978.2004. Epub 2004 Dec 15. J Neurophysiol. 2005. PMID: 15601736
-
Bladder activation by selective stimulation of pudendal nerve afferents in the cat.Exp Neurol. 2008 Jul;212(1):218-25. doi: 10.1016/j.expneurol.2008.04.010. Epub 2008 Apr 20. Exp Neurol. 2008. PMID: 18502417 Free PMC article.
-
Phasic activation of the external urethral sphincter increases voiding efficiency in the rat and the cat.Exp Neurol. 2016 Nov;285(Pt B):173-181. doi: 10.1016/j.expneurol.2016.05.030. Epub 2016 May 25. Exp Neurol. 2016. PMID: 27235934 Free PMC article.
-
Neural control of the urethra.Scand J Urol Nephrol Suppl. 2001;(207):35-43; discussion 106-25. doi: 10.1080/003655901750174872. Scand J Urol Nephrol Suppl. 2001. PMID: 11409613 Review.
-
[Lower urinary tract function and its disorders].Cesk Fysiol. 2000 Aug;49(3):134-44. Cesk Fysiol. 2000. PMID: 11039243 Review. Czech.
Cited by
-
Excitatory and inhibitory effects of stimulation of sacral dorsal root ganglion on bladder reflex in cats.Int Urol Nephrol. 2018 Dec;50(12):2179-2186. doi: 10.1007/s11255-018-2004-9. Epub 2018 Oct 9. Int Urol Nephrol. 2018. PMID: 30302665
-
Stimulation of the pelvic nerve increases bladder capacity in the PGE2 cat model of overactive bladder.Am J Physiol Renal Physiol. 2020 Jun 1;318(6):F1357-F1368. doi: 10.1152/ajprenal.00068.2020. Epub 2020 Apr 20. Am J Physiol Renal Physiol. 2020. PMID: 32308021 Free PMC article.
-
Selective co-stimulation of pudendal afferents enhances reflex bladder activation.Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:1057-60. doi: 10.1109/IEMBS.2011.6090246. Annu Int Conf IEEE Eng Med Biol Soc. 2011. PMID: 22254495 Free PMC article.
-
Improved bladder emptying in urinary retention by electrical stimulation of pudendal afferents.J Neural Eng. 2008 Jun;5(2):144-54. doi: 10.1088/1741-2560/5/2/005. Epub 2008 Apr 22. J Neural Eng. 2008. PMID: 18430976 Free PMC article.
-
Inhibitory Effect and Possible Mechanism of Intraurethral Stimulation on Overactive Bladder in Female Rats.Int Neurourol J. 2015 Sep;19(3):151-7. doi: 10.5213/inj.2015.19.3.151. Epub 2015 Sep 22. Int Neurourol J. 2015. PMID: 26620896 Free PMC article.
References
-
- Balis G, Monroe RR. The pharmacology of chloralose. A review. Psychopharmacologia. 1964;6:1–30. - PubMed
-
- Barrington FJF. The component reflexes of micturition in the cat, Parts I and II. Brain. 1931;54:177–188.
-
- Barrington FJF. The component reflexes of micturition in the cat III. Brain. 1941;64:239–243.
-
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–2697. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

