Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system
- PMID: 16946085
- PMCID: PMC1761128
- DOI: 10.1152/ajpregu.00292.2006
Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system
Abstract
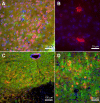
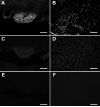
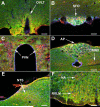
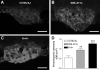
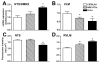
Angiotensin-converting enzyme 2 (ACE2) is a newly discovered carboxy-peptidase responsible for the formation of vasodilatory peptides such as angiotensin-(1-7). We hypothesized that ACE2 is part of the brain renin-angiotensin system, and its expression is regulated by the other elements of this system. ACE2 immunostaining was performed in transgenic mouse brain sections from neuron-specific enolase-AT(1A) (overexpressing AT(1A) receptors), R(+)A(+) (overexpressing angiotensinogen and renin), and control (nontransgenic littermates) mice. Results show that ACE2 staining is widely distributed throughout the brain. Using cell-type-specific antibodies, we observed that ACE2 staining is present in the cytoplasm of neuronal cell bodies but not in glial cells. In the subfornical organ, an area lacking the blood-brain barrier and sensitive to blood-borne angiotensin II, ACE2 was significantly increased in transgenic mice. Interestingly, ACE2 mRNA and protein expression were inversely correlated in the nucleus of tractus solitarius/dorsal motor nucleus of the vagus and the ventrolateral medulla, when comparing transgenic to nontransgenic mice. These results suggest that ACE2 is localized to the cytoplasm of neuronal cells in the brain and that ACE2 levels appear highly regulated by other components of the renin-angiotensin system, confirming its involvement in this system. Moreover, ACE2 expression in brain structures involved in the control of cardiovascular function suggests that the carboxypeptidase may have a role in the central regulation of blood pressure and diseases involving the autonomic nervous system, such as hypertension.
Figures

References
-
- Allen AM, Zhuo J, Mendelsohn FAO. Localization and function of angiotensin AT1 receptors. Am J Hypertens. 2000;13:31S–38S. - PubMed
-
- Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. - PubMed
-
- Bader M, Peters J, Baltatu O, Muller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med. 2001;79:76–102. - PubMed
-
- Banegas I, Prieto I, Alba F, Vives F, Araque A, Segarra AB, Duran R, De Gasparo M, Ramirez M. Angiotensinase activity is asymmetrically distributed in the amygdala, hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2005;156:321–326. - PubMed
-
- Benter IF, Diz DI, Ferrario CM. Pressor and reflex sensitivity is altered in spontaneously hypertensive rats treated with angiotensin-(1-7) Hypertension. 1995;26:1138–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

