Continuous in vivo infusion of interferon-gamma (IFN-gamma) enhances engraftment of syngeneic wild-type cells in Fanca-/- and Fancg-/- mice
- PMID: 16946306
- PMCID: PMC1895443
- DOI: 10.1182/blood-2006-03-007997
Continuous in vivo infusion of interferon-gamma (IFN-gamma) enhances engraftment of syngeneic wild-type cells in Fanca-/- and Fancg-/- mice
Abstract
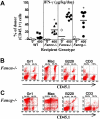
Fanconi anemia (FA) is a heterogeneous genetic disorder characterized by bone marrow (BM) failure and cancer susceptibility. Identification of the cDNAs of FA complementation types allows the potential of using gene transfer technology to introduce functional cDNAs as transgenes into autologous stem cells and provide a cure for the BM failure in FA patients. However, strategies to enhance the mobilization, transduction, and engraftment of exogenous stem cells are required to optimize efficacy prior to widespread clinical use. Hypersensitivity of Fancc-/- cells to interferon-gamma (IFN-gamma), a nongenotoxic immune-regulatory cytokine, enhances engraftment of syngeneic wild-type (WT) cells in Fancc-/- mice. However, whether this phenotype is of broad relevance in other FA complementation groups is unresolved. Here we show that primitive and mature myeloid progenitors in Fanca-/- and Fancg-/- mice are hypersensitive to IFN-gamma and that in vivo infusion of IFN-gamma at clinically relevant concentrations was sufficient to allow consistent long-term engraftment of isogenic WT repopulating stem cells. Given that FANCA, FANCC, and FANCG complementation groups account for more than 90% of all FA patients, these data provide evidence that IFN-gamma conditioning may be a useful nongenotoxic strategy for myelopreparation in FA patients.
Figures


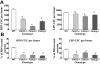

Similar articles
-
Continuous in vivo infusion of interferon-gamma (IFN-gamma) preferentially reduces myeloid progenitor numbers and enhances engraftment of syngeneic wild-type cells in Fancc-/- mice.Blood. 2004 Aug 15;104(4):1204-9. doi: 10.1182/blood-2004-03-1094. Epub 2004 Apr 27. Blood. 2004. PMID: 15113761
-
Evidence for complete epistasis of null mutations in murine Fanconi anemia genes Fanca and Fancg.DNA Repair (Amst). 2011 Dec 10;10(12):1252-61. doi: 10.1016/j.dnarep.2011.09.015. Epub 2011 Oct 28. DNA Repair (Amst). 2011. PMID: 22036606
-
The Fanconi anemia protein FANCF forms a nuclear complex with FANCA, FANCC and FANCG.Hum Mol Genet. 2000 Nov 1;9(18):2665-74. doi: 10.1093/hmg/9.18.2665. Hum Mol Genet. 2000. PMID: 11063725
-
The causes of Fanconi anemia in South Asia and the Middle East: A case series and review of the literature.Mol Genet Genomic Med. 2021 Jul;9(7):e1693. doi: 10.1002/mgg3.1693. Epub 2021 May 7. Mol Genet Genomic Med. 2021. PMID: 33960719 Free PMC article. Review.
-
[Progress of research on protein composition and gene therapy of Fanconi anaemia - review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004 Apr;12(2):231-5. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004. PMID: 15157341 Review. Chinese.
Cited by
-
An abnormal bone marrow microenvironment contributes to hematopoietic dysfunction in Fanconi anemia.Haematologica. 2017 Jun;102(6):1017-1027. doi: 10.3324/haematol.2016.158717. Epub 2017 Mar 24. Haematologica. 2017. PMID: 28341737 Free PMC article.
-
Oxidative stress in Fanconi anemia hematopoiesis and disease progression.Antioxid Redox Signal. 2008 Nov;10(11):1909-21. doi: 10.1089/ars.2008.2129. Antioxid Redox Signal. 2008. PMID: 18627348 Free PMC article. Review.
-
Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence.J Cell Sci. 2007 May 1;120(Pt 9):1572-83. doi: 10.1242/jcs.003152. Epub 2007 Apr 3. J Cell Sci. 2007. PMID: 17405815 Free PMC article.
-
Inflammatory reactive oxygen species-mediated hemopoietic suppression in Fancc-deficient mice.J Immunol. 2007 Apr 15;178(8):5277-87. doi: 10.4049/jimmunol.178.8.5277. J Immunol. 2007. PMID: 17404312 Free PMC article.
-
Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg-/- mice in vivo.Blood. 2009 Mar 5;113(10):2342-51. doi: 10.1182/blood-2008-07-168138. Epub 2009 Jan 7. Blood. 2009. PMID: 19129541 Free PMC article.
References
-
- Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101: 1249-1256. - PubMed
-
- Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86: 2856-2862. - PubMed
-
- Thompson LH. Unraveling the Fanconi anemia-DNA repair connection. Nat Genet. 2005;37: 921-922. - PubMed
-
- Levitus M, Rooimans MA, Steltenpool J, et al. Heterogeneity in Fanconi anemia: evidence for 2 new genetic subtypes. Blood. 2004;103: 2498-2503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

