Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts
- PMID: 16946701
- PMCID: PMC1570424
- DOI: 10.1038/sj.emboj.7601309
Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts
Abstract
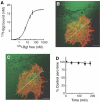
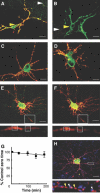
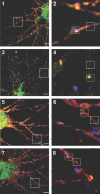
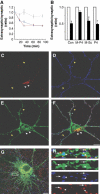
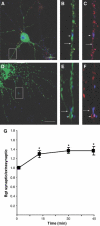
GABAA receptors mediate the majority of fast synaptic inhibition in the brain. The accumulation of these ligand-gated ion channels at synaptic sites is a prerequisite for neuronal inhibition, but the molecular mechanisms underlying this phenomenon remain obscure. To further understand these processes, we have examined the cellular origins of synaptic GABAA receptors. To do so, we have created fluorescent GABAA receptors that are capable of binding -bungarotoxin (Bgt), facilitating the visualization of receptor endocytosis, exocytosis and delivery to synaptic sites. Imaging with Bgt in hippocampal neurons revealed that GABAA receptor endocytosis occurred exclusively at extrasynaptic sites, consistent with the preferential colocalization of extrasynaptic receptors with the AP2 adaptin. Receptor insertion into the plasma membrane was also predominantly extrasynaptic, and pulse-chase analysis revealed that these newly inserted receptors were then able to access directly synaptic sites. Therefore, our results demonstrate that synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. Moreover, they illustrate a dynamic mechanism for neurons to modulate GABAA receptor number at inhibitory synapses by controlling the stability of extrasynaptic receptors.
Figures


References
-
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ (2001) GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci 4: 908–916 - PubMed
-
- Borgdorff AJ, Choquet D (2002) Regulation of AMPA receptor lateral movements. Nature 417: 649–653 - PubMed
-
- Chen ZW, Chang CS, Leil TA, Olcese R, Olsen RW (2005) GABAA receptor-associated protein regulates GABAA receptor cell-surface number in Xenopus laevis oocytes. Mol Pharmacol 68: 152–159 - PubMed
-
- Choquet D, Triller A (2003) The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 4: 251–265 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

