Yersinia has a tropism for B and T cell zones of lymph nodes that is independent of the type III secretion system
- PMID: 16948531
- PMCID: PMC1557584
- DOI: 10.1371/journal.ppat.0020086
Yersinia has a tropism for B and T cell zones of lymph nodes that is independent of the type III secretion system
Abstract
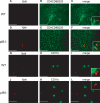
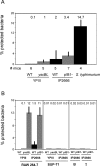
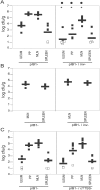
Pathogenic Yersinia have a pronounced tropism for lymphatic tissues and harbor a virulence plasmid that encodes a type III secretion system, pTTSS, that transports Yops into host cells. Yops are critical virulence factors that prevent phagocytosis by macrophages and neutrophils and Yersinia mutants lacking one or more Yops are defective for survival in lymphatic tissues, liver, and gastrointestinal tract. However, here we demonstrate that Y. pseudotuberculosis (Yptb) mutants lacking the pTTSS survived as well as or better than wild-type (WT) Yptb in the mesenteric lymph nodes (MLN). Infection with pTTSS mutants caused lymphadenitis with little necrosis, whereas infection with WT Yptb provoked lymphadenitis with multiple necrotic suppurative foci. Gentamicin protection assays and microscopic examination of the MLN revealed that pTTSS mutants resided extracellularly adjacent to B and T lymphocytes in the cortex and paracortex. WT Yptb was found extracellularly adjacent to neutrophils and macrophages in necrotic areas and adjacent to B and T lymphocytes in less-inflamed areas. To determine whether lymphocytes protected pTTSS mutants from phagocytic cells, Rag1(-/-) mice were infected with pTTSS mutants or WT Yptb. pTTSS mutants but not WT, were impaired for survival in MLN of Rag1(-/-) mice, suggesting that lymphocyte-rich regions constitute a protective niche for pTTSS mutants. Finally, we show that invasin and the chromosomally encoded TTSS were not required for Yptb survival in MLN. In summary, chromosomally encoded factors are sufficient for Yptb replication in the cortex and paracortex of MLN; the pTTSS enables Yersinia to survive within phagocyte-rich areas of lymph nodes, and spread to other tissues.
Conflict of interest statement
Figures




References
-
- Putzker M, Sauer H, Sobe D. Plague and other human infections caused by Yersinia species. Clin Lab. 2001;47:453–466. - PubMed
-
- Smego RA, Frean J, Koornhof HJ. Yersiniosis I: Microbiological and clinicoepidemiological aspects of plague and non-plague Yersinia infections. Eur J Clin Microbiol Infect Dis. 1999;18:1–15. - PubMed
-
- Ibrahim A, Goebel BM, Liesack W, Griffiths M, Stackebrandt E. The phylogeny of the genus Yersinia based on 16S rDNA sequences. FEMS Microbiol Lett. 1993;114:173–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

