A novel mutation in the mitochondrial tRNA(Ser(AGY)) gene associated with mitochondrial myopathy, encephalopathy, and complex I deficiency
- PMID: 16950817
- PMCID: PMC2564579
- DOI: 10.1136/jmg.2005.040626
A novel mutation in the mitochondrial tRNA(Ser(AGY)) gene associated with mitochondrial myopathy, encephalopathy, and complex I deficiency
Abstract
Purpose: To identify molecular defects in a girl with clinical features of MELAS (mitochondrial encephalomyopathy and lactic acidosis) and MERRF (ragged-red fibres) syndromes.
Methods: The enzyme complex activities of the mitochondrial respiratory chain were assayed. Temporal temperature gradient gel electrophoresis was used to scan the entire mitochondrial genome for unknown mitochondrial DNA (mtDNA) alterations, which were then identified by direct DNA sequencing.
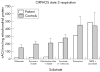
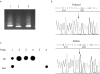
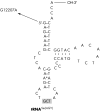
Results: A novel heteroplasmic mtDNA mutation, G12207A, in the tRNA(Ser(AGY)) gene was identified in the patient who had a history of developmental delay, feeding difficulty, lesions within her basal ganglia, cerebral atrophy, proximal muscle weakness, increased blood lactate, liver dysfunction, and fatty infiltration of her muscle. Muscle biopsy revealed ragged red fibres and pleomorphic mitochondria. Study of skeletal muscle mitochondria revealed complex I deficiency associated with mitochondrial proliferation. Real time quantitative PCR analysis showed elevated mtDNA content, 2.5 times higher than normal. The tRNA(Ser(AGY)) mutation was found in heteroplasmic state (92%) in the patient's skeletal muscle. It was not present in her unaffected mother's blood or in 200 healthy controls. This mutation occurs at the first nucleotide of the 5' end of tRNA, which is involved in the formation of the stem region of the amino acid acceptor arm. Mutation at this position may affect processing of the precursor RNA, the stability and amino acid charging efficiency of the tRNA, and overall efficiency of protein translation.
Conclusion: This case underscores the importance of comprehensive mutational analysis of the entire mitochondrial genome when a mtDNA defect is strongly suggested.
Conflict of interest statement
Competing interests: none declared
References
-
- Anderson S, Bankier A T, Barrell B G, de Bruijn M H, Coulson A R, Drouin J, Eperon I C, Nierlich D P, Roe B A, Sanger F, Schreier P H, Smith A J, Staden R, Young I G. Sequence and organization of the human mitochondrial genome. Nature 1981290457–465. - PubMed
-
- Wallace D C. Mitochondrial disease in man and mouse. Science 19992831482–1488. - PubMed
-
- Smeitink J, van den Heuvel L, DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 20012342–352. - PubMed
-
- Wallace D C, Zheng X X, Lott M T, Shoffner J M, Hodge J A, Kelley R I, Epstein C M, Hopkins L C. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell 198855(4)601–610. - PubMed
-
- Pavlakis S G, Phillips P C, DiMauro S, De Vivo D C, Rowland L P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol 198416(4)481–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources