The dps gene of symbiotic "Candidatus Legionella jeonii" in Amoeba proteus responds to hydrogen peroxide and phagocytosis
- PMID: 16950918
- PMCID: PMC1636265
- DOI: 10.1128/JB.00576-06
The dps gene of symbiotic "Candidatus Legionella jeonii" in Amoeba proteus responds to hydrogen peroxide and phagocytosis
Abstract
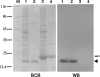
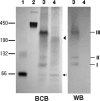
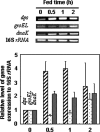
To survive in host cells, intracellular pathogens or symbiotic bacteria require protective mechanisms to overcome the oxidative stress generated by phagocytic activities of the host. By genomic library tagging, we cloned a dps (stands for DNA-binding protein from starved cells) gene of the symbiotic "Candidatus Legionella jeonii" organism (called the X bacterium) (dps(X)) that grows in Amoeba proteus. The gene encodes a 17-kDa protein (pI 5.19) with 91% homology to Dps and DNA-binding ferritin-like proteins of other organisms. The cloned gene complemented the dps mutant of Escherichia coli and conferred resistance to hydrogen peroxide. Dps(X) proteins purified from E. coli transformed with the dps(X) gene were in oligomeric form, formed a complex with pBlueskript SKII DNA, and protected the DNA from DNase I digestion and H(2)O(2)-mediated damage. The expression of the dps(X) gene in "Candidatus Legionella jeonii" was enhanced when the host amoeba was treated with 2 mM H(2)O(2) and by phagocytic activities of the host cell. These results suggested that the Dps protein has a function protective of the bacterial DNA and that its gene expression responds to oxidative stress generated by phagocytic activities of the host cell. With regard to the fact that invasion of Legionella sp. into respiratory phagocytic cells causes pneumonia in mammals, further characterization of dps(X) expression in the Legionella sp. that multiplies in a protozoan host in the natural environment may provide valuable information toward understanding the protective mechanisms of intracellular pathogens.
Figures





Similar articles
-
Phylogenetic characterization of Legionella-like endosymbiotic X-bacteria in Amoeba proteus: a proposal for 'Candidatus Legionella jeonii' sp. nov.Environ Microbiol. 2004 Dec;6(12):1252-63. doi: 10.1111/j.1462-2920.2004.00659.x. Environ Microbiol. 2004. PMID: 15560823
-
Characterization of a cDNA of peroxiredoxin II responding to hydrogen peroxide and phagocytosis in Amoeba proteus.J Eukaryot Microbiol. 2005 May-Jun;52(3):223-30. doi: 10.1111/j.1550-7408.2005.00027.x. J Eukaryot Microbiol. 2005. PMID: 15926998
-
Structural studies on the second Mycobacterium smegmatis Dps: invariant and variable features of structure, assembly and function.J Mol Biol. 2008 Jan 25;375(4):948-59. doi: 10.1016/j.jmb.2007.10.023. Epub 2007 Oct 16. J Mol Biol. 2008. PMID: 18061613
-
The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding.Biochim Biophys Acta. 2010 Aug;1800(8):798-805. doi: 10.1016/j.bbagen.2010.01.013. Epub 2010 Feb 4. Biochim Biophys Acta. 2010. PMID: 20138126 Review.
-
Role of Dps (DNA-binding proteins from starved cells) aggregation on DNA.Front Biosci (Landmark Ed). 2010 Jan 1;15(1):122-31. doi: 10.2741/3610. Front Biosci (Landmark Ed). 2010. PMID: 20036810 Review.
Cited by
-
An Overview of Dps: Dual Acting Nanovehicles in Prokaryotes with DNA Binding and Ferroxidation Properties.Subcell Biochem. 2021;96:177-216. doi: 10.1007/978-3-030-58971-4_3. Subcell Biochem. 2021. PMID: 33252729 Review.
-
Unity in variety--the pan-genome of the Chlamydiae.Mol Biol Evol. 2011 Dec;28(12):3253-70. doi: 10.1093/molbev/msr161. Epub 2011 Jun 20. Mol Biol Evol. 2011. PMID: 21690563 Free PMC article.
-
DNA-mediated charge transport in redox sensing and signaling.J Am Chem Soc. 2010 Jan 27;132(3):891-905. doi: 10.1021/ja907669c. J Am Chem Soc. 2010. PMID: 20047321 Free PMC article.
-
The OxyR regulon in nontypeable Haemophilus influenzae.J Bacteriol. 2007 Feb;189(3):1004-12. doi: 10.1128/JB.01040-06. Epub 2006 Dec 1. J Bacteriol. 2007. PMID: 17142400 Free PMC article.
-
A ferritin-like protein with antioxidant activity in Ureaplasma urealyticum.BMC Microbiol. 2015 Jul 26;15:145. doi: 10.1186/s12866-015-0485-6. BMC Microbiol. 2015. PMID: 26209240 Free PMC article.
References
-
- Ahn, T. I., S. T. Lim, H. K. Leeu, J. E. Lee, and K. W. Jeon. 1994. A novel strong promoter of the groEx operon of symbiotic bacteria in Amoeba proteus. Gene 128:43-49. - PubMed
-
- Almirón, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. - PubMed
-
- Andrews, S. C., A. K. Robinson, and F. Rodríguez-Quiñones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. - PubMed
-
- Babior, B. M. 2000. Phagocytes and oxidative stress. Am. J. Med. 109:33-44. - PubMed
-
- Babior, B. M. 1978. Oxygen-dependent microbial killing by phagocytes. N. Engl. J. Med. 298:645-721. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

