Posttranslational modification of the 20S proteasomal proteins of the archaeon Haloferax volcanii
- PMID: 16950923
- PMCID: PMC1636277
- DOI: 10.1128/JB.00943-06
Posttranslational modification of the 20S proteasomal proteins of the archaeon Haloferax volcanii
Abstract
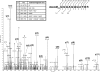
20S proteasomes are large, multicatalytic proteases that play an important role in intracellular protein degradation. The barrel-like architecture of 20S proteasomes, formed by the stacking of four heptameric protein rings, is highly conserved from archaea to eukaryotes. The outer two rings are composed of alpha-type subunits, and the inner two rings are composed of beta-type subunits. The halophilic archaeon Haloferax volcanii synthesizes two different alpha-type proteins, alpha1 and alpha2, and one beta-type protein that assemble into at least two 20S proteasome subtypes. In this study, we demonstrate that all three of these 20S proteasomal proteins (alpha1, alpha2, and beta) are modified either post- or cotranslationally. Using electrospray ionization quadrupole time-of-flight mass spectrometry, a phosphorylation site of the beta subunit was identified at Ser129 of the deduced protein sequence. In addition, alpha1 and alpha2 contained N-terminal acetyl groups. These findings represent the first evidence of acetylation and phosphorylation of archaeal proteasomes and are one of the limited examples of post- and/or cotranslational modification of proteins in this unusual group of organisms.
Figures
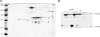



Similar articles
-
Phosphorylation and methylation of proteasomal proteins of the haloarcheon Haloferax volcanii.Archaea. 2010 Jul 8;2010:481725. doi: 10.1155/2010/481725. Archaea. 2010. PMID: 20671954 Free PMC article.
-
Halophilic 20S proteasomes of the archaeon Haloferax volcanii: purification, characterization, and gene sequence analysis.J Bacteriol. 1999 Sep;181(18):5814-24. doi: 10.1128/JB.181.18.5814-5824.1999. J Bacteriol. 1999. PMID: 10482525 Free PMC article.
-
Chemical cross-linking, mass spectrometry, and in silico modeling of proteasomal 20S core particles of the haloarchaeon Haloferax volcanii.Proteomics. 2012 Jun;12(11):1806-14. doi: 10.1002/pmic.201100260. Proteomics. 2012. PMID: 22623373 Free PMC article.
-
Comprehensive mass spectrometric analysis of the 20S proteasome complex.Methods Enzymol. 2005;405:187-236. doi: 10.1016/S0076-6879(05)05009-3. Methods Enzymol. 2005. PMID: 16413316 Review.
-
Add salt, add sugar: N-glycosylation in Haloferax volcanii.Biochem Soc Trans. 2013 Feb 1;41(1):432-5. doi: 10.1042/BST20120142. Biochem Soc Trans. 2013. PMID: 23356324 Review.
Cited by
-
Functional genomic and advanced genetic studies reveal novel insights into the metabolism, regulation, and biology of Haloferax volcanii.Archaea. 2011;2011:602408. doi: 10.1155/2011/602408. Epub 2011 Nov 30. Archaea. 2011. PMID: 22190865 Free PMC article.
-
Ser/Thr/Tyr protein phosphorylation in the archaeon Halobacterium salinarum--a representative of the third domain of life.PLoS One. 2009;4(3):e4777. doi: 10.1371/journal.pone.0004777. Epub 2009 Mar 10. PLoS One. 2009. PMID: 19274099 Free PMC article.
-
Proteasomes and protein conjugation across domains of life.Nat Rev Microbiol. 2011 Dec 19;10(2):100-11. doi: 10.1038/nrmicro2696. Nat Rev Microbiol. 2011. PMID: 22183254 Free PMC article. Review.
-
The complete genome sequence of Haloferax volcanii DS2, a model archaeon.PLoS One. 2010 Mar 19;5(3):e9605. doi: 10.1371/journal.pone.0009605. PLoS One. 2010. PMID: 20333302 Free PMC article.
-
Extreme challenges and advances in archaeal proteomics.Curr Opin Microbiol. 2012 Jun;15(3):351-6. doi: 10.1016/j.mib.2012.02.002. Epub 2012 Mar 1. Curr Opin Microbiol. 2012. PMID: 22386447 Free PMC article. Review.
References
-
- Bab-Dinitz, E., H. Shmuely, J. Maupin-Furlow, J. Eichler, and B. Shaanan. 2006. Haloferax volcanii PitA: an example of functional interaction between the Pfam chlorite dismutase and antibiotic biosynthesis monooxygenase families? Bioinformatics 22:671-675. - PubMed
-
- Baumeister, W., J. Walz, F. Zühl, and E. Seemüller. 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367-380. - PubMed
-
- Bell, S. D., C. H. Botting, B. N. Wardleworth, S. P. Jackson, and M. F. White. 2002. The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science 296:148-151. - PubMed
-
- Benaroudj, N., P. Zwickl, E. Seemuller, W. Baumeister, and A. L. Goldberg. 2003. ATP hydrolysis by the proteasome regulatory complex PAN serves multiple functions in protein degradation. Mol. Cell 11:69-78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

