TOS9 regulates white-opaque switching in Candida albicans
- PMID: 16950924
- PMCID: PMC1595353
- DOI: 10.1128/EC.00252-06
TOS9 regulates white-opaque switching in Candida albicans
Abstract
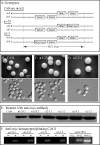
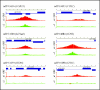
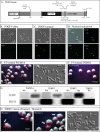
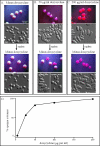
In Candida albicans, the a1-alpha2 complex represses white-opaque switching, as well as mating. Based upon the assumption that the a1-alpha2 corepressor complex binds to the gene that regulates white-opaque switching, a chromatinimmunoprecipitation-microarray analysis strategy was used to identify 52 genes that bound to the complex. One of these genes, TOS9, exhibited an expression pattern consistent with a "master switch gene." TOS9 was only expressed in opaque cells, and its gene product, Tos9p, localized to the nucleus. Deletion of the gene blocked cells in the white phase, misexpression in the white phase caused stable mass conversion of cells to the opaque state, and misexpression blocked temperature-induced mass conversion from the opaque state to the white state. A model was developed for the regulation of spontaneous switching between the opaque state and the white state that includes stochastic changes of Tos9p levels above and below a threshold that induce changes in the chromatin state of an as-yet-unidentified switching locus. TOS9 has also been referred to as EAP2 and WOR1.
Figures


References
-
- Bennett, R. J., and A. D. Johnson. 2005. Mating in Candida albicans and the search for a sexual cycle. Annu. Rev. Microbiol. 59:233-255. - PubMed
-
- Bergen, M., E. Voss, and D. R. Soll. 1990. Switching at the cellular level in the white-opaque transition of Candida albicans. J. Gen. Microbiol. 136:1925-1936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

