The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells
- PMID: 16951258
- PMCID: PMC1560419
- DOI: 10.1101/gad.381806
The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells
Abstract
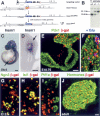
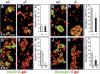
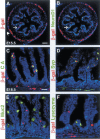
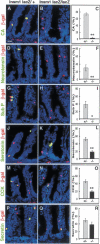
The pancreatic and intestinal primordia contain epithelial progenitor cells that generate many cell types. During development, specific programs of gene expression restrict the developmental potential of such progenitors and promote their differentiation. The Insm1 (insulinoma-associated 1, IA-1) gene encodes a Zinc-finger factor that was discovered in an insulinoma cDNA library. We show that pancreatic and intestinal endocrine cells express Insm1 and require Insm1 for their development. In the pancreas of Insm1 mutant mice, endocrine precursors are formed, but only few insulin-positive beta cells are generated. Instead, endocrine precursor cells accumulate that express none of the pancreatic hormones. A similar change is observed in the development of intestine, where endocrine precursor cells are formed but do not differentiate correctly. A hallmark of endocrine cell differentiation is the accumulation of proteins that participate in secretion and vesicle transport, and we find many of the corresponding genes to be down-regulated in Insm1 mutant mice. Insm1 thus controls a gene expression program that comprises hormones and proteins of the secretory machinery. Our genetic analysis has revealed a key role of Insm1 in differentiation of pancreatic and intestinal endocrine cells.
Figures


References
-
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D.J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. - PubMed
-
- Artner I., Le Lay J., Hang Y., Elghazi L., Schisler J.C., Henderson E., Sosa-Pineda B., Stein R. MafB: An activator of the glucagon gene expressed in developing islet α-and β-cells. Diabetes. 2006;55:297–304. - PubMed
-
- Bell G.I., Polonsky K.S. Diabetes mellitus and genetically programmed defects in β-cell function. Nature. 2001;414:788–791. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
