Gliding motility of Mycoplasma mobile can occur by repeated binding to N-acetylneuraminyllactose (sialyllactose) fixed on solid surfaces
- PMID: 16952936
- PMCID: PMC1595466
- DOI: 10.1128/JB.00754-06
Gliding motility of Mycoplasma mobile can occur by repeated binding to N-acetylneuraminyllactose (sialyllactose) fixed on solid surfaces
Abstract
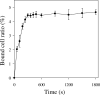
Mycoplasma mobile relies on an unknown mechanism to glide across solid surfaces including glass, animal cells, and plastics. To identify the direct binding target, we examined the factors that affect the binding of Mycoplasma pneumoniae to solid surfaces and concluded that N-acetylneuraminyllactose (sialyllactose) attached to a protein can mediate glass binding on the basis of the following four lines of evidence: (i) glass binding was inhibited by N-acetylneuraminidase, (ii) glass binding was inhibited by N-acetylneuraminyllactose in a structure-dependent manner, (iii) binding occurred on glass pretreated with bovine serum albumin attached to N-acetylneuraminyllactose, and (iv) gliding speed depended on the density of N-acetylneuraminyllactose on glass.
Figures

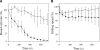



Similar articles
-
Sialylated Receptor Setting Influences Mycoplasma pneumoniae Attachment and Gliding Motility.Mol Microbiol. 2018 Sep;109(6):735-744. doi: 10.1111/mmi.13997. Epub 2018 Sep 30. Mol Microbiol. 2018. PMID: 29885004 Free PMC article.
-
Gliding Motility of Mycoplasma mobile on Uniform Oligosaccharides.J Bacteriol. 2015 Sep;197(18):2952-7. doi: 10.1128/JB.00335-15. Epub 2015 Jul 6. J Bacteriol. 2015. PMID: 26148712 Free PMC article.
-
Behaviors and Energy Source of Mycoplasma gallisepticum Gliding.J Bacteriol. 2019 Sep 6;201(19):e00397-19. doi: 10.1128/JB.00397-19. Print 2019 Oct 1. J Bacteriol. 2019. PMID: 31308069 Free PMC article.
-
[Gliding motility of mycoplasmas, a novel mechanism of biological motility].Tanpakushitsu Kakusan Koso. 2005 Mar;50(3):239-45. Tanpakushitsu Kakusan Koso. 2005. PMID: 15773304 Review. Japanese. No abstract available.
-
Unique centipede mechanism of Mycoplasma gliding.Annu Rev Microbiol. 2010;64:519-37. doi: 10.1146/annurev.micro.112408.134116. Annu Rev Microbiol. 2010. PMID: 20533876 Review.
Cited by
-
Motility Assays of Mycoplasma mobile Under Light Microscopy.Methods Mol Biol. 2023;2646:321-325. doi: 10.1007/978-1-0716-3060-0_26. Methods Mol Biol. 2023. PMID: 36842126
-
Mycoplasma mobile cells elongated by detergent and their pivoting movements in gliding.J Bacteriol. 2012 Jan;194(1):122-30. doi: 10.1128/JB.05857-11. Epub 2011 Oct 14. J Bacteriol. 2012. PMID: 22001513 Free PMC article.
-
Cytoskeletal asymmetrical dumbbell structure of a gliding mycoplasma, Mycoplasma gallisepticum, revealed by negative-staining electron microscopy.J Bacteriol. 2009 May;191(10):3256-64. doi: 10.1128/JB.01823-08. Epub 2009 Mar 13. J Bacteriol. 2009. PMID: 19286806 Free PMC article.
-
Refined Mechanism of Mycoplasma mobile Gliding Based on Structure, ATPase Activity, and Sialic Acid Binding of Machinery.mBio. 2019 Dec 24;10(6):e02846-19. doi: 10.1128/mBio.02846-19. mBio. 2019. PMID: 31874918 Free PMC article.
-
Force and Stepwise Movements of Gliding Motility in Human Pathogenic Bacterium Mycoplasma pneumoniae.Front Microbiol. 2021 Sep 24;12:747905. doi: 10.3389/fmicb.2021.747905. eCollection 2021. Front Microbiol. 2021. PMID: 34630372 Free PMC article.
References
-
- Aluotto, B. B., R. G. Wittler, C. O. Williams, and J. E. Faber. 1970. Standardized bacteriologic techniques for the characterization of mycoplasma species. Int. J. Syst. Bacteriol. 20:35-58.
-
- Bredt, W. 1979. Motility, p. 141-145. In M. F. Barile, S. Razin, J. G. Tully, and R. F. Whitcomb (ed.), The mycoplasmas, vol. 1. Academic Press, New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

