Expression of the primary carbohydrate component of the Bordetella bronchiseptica biofilm matrix is dependent on growth phase but independent of Bvg regulation
- PMID: 16952960
- PMCID: PMC1595472
- DOI: 10.1128/JB.00605-06
Expression of the primary carbohydrate component of the Bordetella bronchiseptica biofilm matrix is dependent on growth phase but independent of Bvg regulation
Abstract
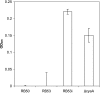
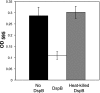
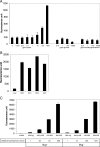
We previously showed that the Bvg virulence control system regulates biofilm formation in Bordetella bronchiseptica (Y. Irie, S. Mattoo, and M. H. Yuk, J. Bacteriol. 186:5692-5698, 2004). Analyses of the extracellular components of B. bronchiseptica biofilm matrix revealed that the major sugar component in the matrix was xylose, and linkage analysis indicated a majority of it to be in a 4-linked polymeric form. The production of xylose was independent of Bvg regulation but instead was dependent on bacterial growth phase. In addition, N-acetyl-glucosamine in the matrix was found to be important for the initial development of the biofilm. These results suggest that B. bronchiseptica biofilm formation is growth phase dependent in addition to being regulated by the Bvg virulence system.
Figures

Similar articles
-
The Bvg virulence control system regulates biofilm formation in Bordetella bronchiseptica.J Bacteriol. 2004 Sep;186(17):5692-8. doi: 10.1128/JB.186.17.5692-5698.2004. J Bacteriol. 2004. PMID: 15317773 Free PMC article.
-
The bvg-repressed gene brtA, encoding biofilm-associated surface adhesin, is expressed during host infection by Bordetella bronchiseptica.Microbiol Immunol. 2016 Feb;60(2):93-105. doi: 10.1111/1348-0421.12356. Microbiol Immunol. 2016. PMID: 26756546
-
The BvgAS signal transduction system regulates biofilm development in Bordetella.J Bacteriol. 2005 Feb;187(4):1474-84. doi: 10.1128/JB.187.4.1474-1484.2005. J Bacteriol. 2005. PMID: 15687212 Free PMC article.
-
Outer membrane protein OmpQ of Bordetella bronchiseptica is required for mature biofilm formation.Microbiology (Reading). 2016 Feb;162(2):351-363. doi: 10.1099/mic.0.000224. Epub 2015 Dec 15. Microbiology (Reading). 2016. PMID: 26673448
-
Genetic regulation of airway colonization by Bordetella species.Am J Respir Crit Care Med. 1996 Oct;154(4 Pt 2):S150-4. doi: 10.1164/ajrccm/154.4_Pt_2.S150. Am J Respir Crit Care Med. 1996. PMID: 8876534 Review.
Cited by
-
Bordetella Pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance.Med Microbiol Immunol. 2018 Feb;207(1):3-26. doi: 10.1007/s00430-017-0524-z. Epub 2017 Nov 21. Med Microbiol Immunol. 2018. PMID: 29164393 Review.
-
BpsR modulates Bordetella biofilm formation by negatively regulating the expression of the Bps polysaccharide.J Bacteriol. 2012 Jan;194(2):233-42. doi: 10.1128/JB.06020-11. Epub 2011 Nov 4. J Bacteriol. 2012. PMID: 22056934 Free PMC article.
-
Aggregatibacter actinomycetemcomitans Dispersin B: The Quintessential Antibiofilm Enzyme.Pathogens. 2024 Aug 7;13(8):668. doi: 10.3390/pathogens13080668. Pathogens. 2024. PMID: 39204268 Free PMC article. Review.
-
Microarray and functional analysis of growth phase-dependent gene regulation in Bordetella bronchiseptica.Infect Immun. 2009 Oct;77(10):4221-31. doi: 10.1128/IAI.00136-09. Epub 2009 Aug 10. Infect Immun. 2009. PMID: 19667046 Free PMC article.
-
The Bordetella Bps polysaccharide is critical for biofilm development in the mouse respiratory tract.J Bacteriol. 2007 Nov;189(22):8270-6. doi: 10.1128/JB.00785-07. Epub 2007 Jun 22. J Bacteriol. 2007. PMID: 17586629 Free PMC article.
References
-
- Allison, D. G., B. Ruiz, C. SanJose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. - PubMed
-
- Branda, S. S., S. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. - PubMed
-
- Cotter, P. A., and A. M. Jones. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11:367-373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

