FliT acts as an anti-FlhD2C2 factor in the transcriptional control of the flagellar regulon in Salmonella enterica serovar typhimurium
- PMID: 16952964
- PMCID: PMC1595477
- DOI: 10.1128/JB.00799-06
FliT acts as an anti-FlhD2C2 factor in the transcriptional control of the flagellar regulon in Salmonella enterica serovar typhimurium
Abstract
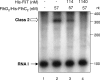
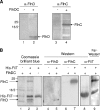
Flagellar operons are divided into three classes with respect to their transcriptional hierarchy in Salmonella enterica serovar Typhimurium. The class 1 gene products FlhD and FlhC act together in an FlhD(2)C(2) heterotetramer, which binds upstream of the class 2 promoters to facilitate binding of RNA polymerase. Class 2 expression is known to be enhanced by a disruption mutation in a flagellar gene, fliT. In this study, we purified FliT protein in a His-tagged form and showed that the protein prevented binding of FlhD(2)C(2) to the class 2 promoter and inhibited FlhD(2)C(2)-dependent transcription. Pull-down and far-Western blotting analyses revealed that the FliT protein was capable of binding to FlhD(2)C(2) and FlhC and not to FlhD alone. We conclude that FliT acts as an anti-FlhD(2)C(2) factor, which binds to FlhD(2)C(2) through interaction with the FlhC subunit and inhibits its binding to the class 2 promoter.
Figures

References
-
- Aldridge, P., J. Karlinsey, and K. T. Hughes. 2003. The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol. Microbiol. 49:1333-1345. - PubMed
-
- Amann, E., B. Ochs, and K.-J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. - PubMed
-
- Claret, L., and C. Hughes. 2000. Functions of the subunits in the FlhD2C2 transcriptional master regulator of bacterial flagellum biogenesis and swarming. J. Mol. Biol. 303:467-478. - PubMed
-
- Claret, L., and C. Hughes. 2002. Interaction of the atypical prokaryotic transcription activator FlhD2C2 with early promoters of the flagellar gene hierarchy. J. Mol. Biol. 321:185-199. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

