Induced chirality of the light-harvesting carotenoid salinixanthin and its interaction with the retinal of xanthorhodopsin
- PMID: 16953586
- PMCID: PMC2528006
- DOI: 10.1021/bi061098i
Induced chirality of the light-harvesting carotenoid salinixanthin and its interaction with the retinal of xanthorhodopsin
Abstract
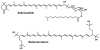
In xanthorhodopsin, a retinal protein-carotenoid complex of Salinibacter ruber, the carotenoid salinixanthin functions as a light-harvesting antenna in supplying additional excitation energy for retinal isomerization and proton transport. Another retinal protein, archaerhodopsin, has been shown to contain a carotenoid, bacterioruberin, but without an antenna function. We report here that the binding site confers a chiral geometry on salinixanthin in xanthorhodopsin and confirm that the same is true for bacterioruberin in archaerhodopsin. Cell membranes containing these rhodopsins exhibit CD spectra with sharp positive bands in the visible region where the carotenoids absorb, and in the case of xanthorhodopsin a negative band at 536 nm, as well as bands in the UV region. The carotenoid in ethanol has very weak optical activity in the visible region of the spectrum. Denaturation of the opsin upon deprotonation of the Schiff base at pH 12.5 eliminates the induced CD bands in both proteins. In one of these proteins, but not in the other, the carotenoid binding site depends entirely on the retinal. Hydrolysis of the retinal Schiff base of xanthorhodopsin with hydroxylamine eliminates the induced CD bands of salinixanthin. In contrast, hydrolysis of the Schiff base in archaerhodopsin does not abolish the CD bands of bacterioruberin. Thus, consistent with its antenna function, the carotenoid binding site interacts closely with the retinal only in xanthorhodopsin, and this interaction is the major source of the CD bands. In this protein, protonation of the counterion with a decrease in pH from 8 to 5 causes significant changes in the CD spectrum. The observed spectral features suggest that binding of salinixanthin in xanthorhodopsin involves the cyclohexenone ring of the carotenoid and its conformational heterogeneity is restricted.
Figures





References
-
- Antón J, Oren A, Benlloch S, Rodríguez-Valera F, Amann R, Rosselló-Mora R. Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int. J. Syst. Evol. Micrbiol. 2002;52:485–491. - PubMed
-
- Lutnaes BF, Oren A, Liaaen-Jensen S. New C-40-carotenoid acyl glycoside as principal carotenoid in Salinibacter ruber, an extremely halophilic eubacterium. J. Nat. Prod. 2002;65:1340–1343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

