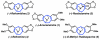
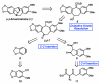
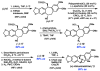
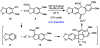A convergent and enantioselective synthesis of (+)-amurensinine via selective C-H and C-C bond insertion reactions
- PMID: 16953603
- PMCID: PMC2529249
- DOI: 10.1021/ja0651815
A convergent and enantioselective synthesis of (+)-amurensinine via selective C-H and C-C bond insertion reactions
Abstract
A convergent and enantioselective synthesis of the natural product amurensinine is described. The synthetic strategy takes advantage of mild and selective C-H and C-C bond insertion reactions, in addition to the palladium-catalyzed aerobic oxidative kinetic resolution recently developed in these laboratories.
Figures
Similar articles
-
Palladium-catalyzed enantioselective oxidation of chiral secondary alcohols: access to both enantiomeric series.Angew Chem Int Ed Engl. 2008;47(34):6367-70. doi: 10.1002/anie.200801865. Angew Chem Int Ed Engl. 2008. PMID: 18626882 Free PMC article. No abstract available.
-
Pd-catalyzed enantioselective aerobic oxidation of secondary alcohols: applications to the total synthesis of alkaloids.J Am Chem Soc. 2008 Oct 15;130(41):13745-54. doi: 10.1021/ja804738b. Epub 2008 Sep 18. J Am Chem Soc. 2008. PMID: 18798630 Free PMC article.
-
Synthesis of Methylene-Bridged Biscarbazole Alkaloids by using an Ullmann-type Coupling: First Total Synthesis of Murrastifoline-C and Murrafoline-E.Chemistry. 2016 Feb 12;22(7):2487-500. doi: 10.1002/chem.201504680. Epub 2016 Jan 20. Chemistry. 2016. PMID: 26787133
-
Synthesis of pyrrole and carbazole alkaloids.Top Curr Chem. 2012;309:203-53. doi: 10.1007/128_2011_192. Top Curr Chem. 2012. PMID: 21728136 Review.
-
Enantioselective palladium-catalyzed allylic alkylation reactions in the synthesis of Aspidosperma and structurally related monoterpene indole alkaloids.Nat Prod Rep. 2018 Jun 20;35(6):559-574. doi: 10.1039/c7np00069c. Nat Prod Rep. 2018. PMID: 29658039 Free PMC article. Review.
Cited by
-
An efficient synthesis of 4-phenoxy-quinazoline, 2-phenoxy-quinoxaline, and 2-phenoxy-pyridine derivatives using aryne chemistry.RSC Adv. 2021 Jan 15;11(6):3477-3483. doi: 10.1039/d0ra09994e. eCollection 2021 Jan 14. RSC Adv. 2021. PMID: 35424287 Free PMC article.
-
Recent Advances in the Total Synthesis of the Tetrahydroisoquinoline Alkaloids (2002-2020).Chem Rev. 2023 Aug 9;123(15):9447-9496. doi: 10.1021/acs.chemrev.3c00054. Epub 2023 Jul 10. Chem Rev. 2023. PMID: 37429001 Free PMC article. Review.
-
Recent Applications of Imines as Key Intermediates in the Synthesis of Alkaloids and Novel Nitrogen Heterocycles.Pure Appl Chem. 2009;81(2):195-204. doi: 10.1351/PAC-CON-08-07-03. Pure Appl Chem. 2009. PMID: 20046937 Free PMC article.
-
Concise enantiospecific total synthesis of tubingensin A.J Am Chem Soc. 2014 Feb 26;136(8):3036-9. doi: 10.1021/ja501142e. Epub 2014 Feb 18. J Am Chem Soc. 2014. PMID: 24524351 Free PMC article.
-
Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings.Nat Commun. 2016 Dec 22;7:13852. doi: 10.1038/ncomms13852. Nat Commun. 2016. PMID: 28004746 Free PMC article.
References
-
- Kakiuchi F, Murai S. Topics in Organometallic Chemistry Series. In: Murai S, editor. Activation of Unreactive Bonds and Organic Synthesis. Vol. 3. Springer-Verlag; Berlin and New York: 1999. pp. 47–79.
- Murakami M, Ito Y. Topics in Organometallic Chemistry Series. In: Murai S, editor. Activation of Unreactive Bonds and Organic Synthesis. Vol. 3. Springer-Verlag; Berlin and New York: 1999. pp. 97–129.
- Crabtree RH. Chem. Rev. 1985;85:245–269.
- Labinger JA, Bercaw JE. Nature. 2002;417:507–514. - PubMed
- Davies HML, Beckwith REJ. Chem. Rev. 2003;103:2861–2903. - PubMed
-
-
For recent examples, see: Davies HML, Dai X, Long MS. J. Am. Chem. Soc. 2006;128:2485–2490.O'Malley SJ, Tan KL, Watzke A, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2005;127:13496–13497.Kurosawa W, Kobayashi H, Kan T, Fukuyama T. Tetrahedron. 2004;60:9615–9628.Wehn PM, Du Bois J. J. Am. Chem. Soc. 2002;124:12950–12951.Johnson JA, Li N, Sames D. J. Am. Chem. Soc. 2002;124:6900–6903.
-
-
- Boit HG, Flentje H. Naturwiss. 1960;47:180.
- Gözler B, Lantz MS, Shamma M. J. Nat. Prod. 1983;46:293–309.
-
-
For selected syntheses of isopavine alkaloids, see: Gözler B. Pavine and Isopavine Alkaloids. In: Brossi A, editor. The Alkaloids. Vol. 31. Academic Press; New York: 1987. pp. 343–356.Shinohara T, Takeda A, Toda J, Sano T. Heterocycles. 1998;48:981–992.Gottlieb L, Meyers AIJ. Org. Chem. 1990;55:5659–5662.Hanessian S, Mauduit M. Angew. Chem., Int. Ed. 2001;40:3810–3813.Dragoli DR, Burdett MT, Ellman JA. J. Am. Chem. Soc. 2001;123:10127–10128.
-
-
-
For discussions on the biological activity of the isopavine alkaloids, see: Weber E, Keana J, Barmettler P. PCT Int. Appl. WO 12575, 1990. Chem. Abstr. 1991;115:106019w.Childers WE, Abou-Gharbia MA. Chem. Abstr. U.S. Patent 4940789, 1990 1990. p. 191190w.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources