Total synthesis of piericidin A1 and B1 and key analogues
- PMID: 16953619
- PMCID: PMC2531196
- DOI: 10.1021/ja0632862
Total synthesis of piericidin A1 and B1 and key analogues
Abstract
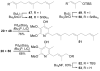
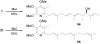
Full details of the total synthesis of piericidin A1 and B1 and its extension to the preparation of a series of key analogues are described including ent-piericidin A1 (ent-1), 4'-deshydroxypiericidin A1 (58), 5'-desmethylpiericidin A1 (73), 4'-deshydroxy-5'-desmethylpiericidin A1 (75), and the corresponding analogues 51, 59, 76, and 77 bearing a simplified farnesyl side chain. The evaluation of these key analogues, along with those derived from their further functionalizations, permitted a scan of the key structural features providing new insights into the role of the substituents found in both the pyridyl core as well as the side chain. A strategic late stage heterobenzylic Stille cross-coupling reaction of the pyridyl core with the fully elaborated side chain permitted ready access to the analogues in which each half of the molecule could be systematically and divergently modified. The pyridyl cores were assembled enlisting inverse electron demand Diels-Alder reactions of N-sulfonyl-1-azabutadienes, while key elements of side chain syntheses include an anti selective asymmetric aldol to install the C9 and C10 relative and absolute stereochemistry (for natural and ent-1) and a modified Julia olefination for formation of the C5-C6 trans double bond with convergent assemblage of the side chains.
Figures














References
-
- Takahashi N, Suzuki A, Tamura S. J. Am. Chem. Soc. 1965;87:2066. - PubMed
-
- Nelson DL, Cox MM. Lenhinger Principles of Biochemistry. Worth; New York: 2000. Chapter 19.
-
- Esposti MD. Biochim. Biophys. Acta. 1998;1364:222. - PubMed
-
- Yoshida S, Yoneyama K, Shiraishi S, Watanbe A, Takahashi N. Agric. Biol. Chem. 1977;41:849.
-
- Kimura K, Takahashi H, Miyata N, Yoshihama M, Uramoto M. J. Antibiot. 1996;49:697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

