Methodological issues in the assessment of antimalarial drug treatment: analysis of 13 studies in eight African countries from 2001 to 2004
- PMID: 16954313
- PMCID: PMC1635238
- DOI: 10.1128/AAC.01618-05
Methodological issues in the assessment of antimalarial drug treatment: analysis of 13 studies in eight African countries from 2001 to 2004
Abstract
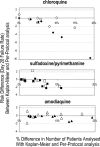
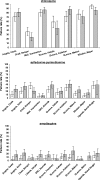
The objectives of these analyses were to assess the feasibility of the latest WHO recommendations (28-day follow-up with PCR genotyping) for the assessment of antimalarial drug efficacy in vivo and to examine how different statistical approaches affect results. We used individual-patient data from 13 studies of uncomplicated pediatric falciparum malaria conducted in sub-Saharan Africa, using chloroquine (CQ), sulfadoxine/pyrimethamine (SP), or amodiaquine (AQ). We assessed the use effectiveness and test performance of PCR genotyping in distinguishing recurrent infections. In analyzing data, we compared (i) the risk of failure on target days (days 14 and 28) by using Kaplan-Meier and per-protocol evaluable patient analyses, (ii) PCR-corrected results allowing (method 1) or excluding (method 2) new infections, (iii) and day 14 versus day 28 results. Of the 2,576 patients treated, 2,287 (89%) were evaluable on day 28. Of the 695 recurrences occurring post-day 14, 650 could be processed and 584 were resolved (PCR use effectiveness, 84%; test performance, 90%). The risks of failure on day 28 with Kaplan-Meier and evaluable-patient analyses tended to be generally close (except in smaller studies) because the numbers of dropouts were minimal, but attrition rates on day 28 were higher with the latter method. Method 2 yielded higher risks of failure than method 1. Extending observation to 28 days produced higher estimated risks of failure for SP and AQ but not for CQ (high failure rates by day 14). Results support the implementation of the current WHO protocol and favor analyzing PCR-corrected outcomes by Kaplan-Meier analysis (which allows for dropouts) and retaining new infections (which minimizes losses).
Figures
Similar articles
-
Low efficacy of amodiaquine or chloroquine plus sulfadoxine-pyrimethamine against Plasmodium falciparum and P. vivax malaria in Papua New Guinea.Am J Trop Med Hyg. 2007 Nov;77(5):947-54. Am J Trop Med Hyg. 2007. PMID: 17984359 Clinical Trial.
-
Improved efficacy with amodiaquine instead of chloroquine in sulfadoxine/pyrimethamine combination treatment of falciparum malaria in Uganda: experience with fixed-dose formulation.Acta Trop. 2006 Nov;100(1-2):142-50. doi: 10.1016/j.actatropica.2006.10.007. Epub 2006 Nov 20. Acta Trop. 2006. PMID: 17113554 Clinical Trial.
-
Therapeutic efficacy of sulfadoxine-pyrimethamine, amodiaquine and the sulfadoxine-pyrimethamine-amodiaquine combination against uncomplicated Plasmodium falciparum malaria in young children in Cameroon.Bull World Health Organ. 2002;80(7):538-45. Bull World Health Organ. 2002. PMID: 12163917 Free PMC article. Clinical Trial.
-
Antimalarial efficacy of sulfadoxine-pyrimethamine, amodiaquine and a combination of chloroquine plus sulfadoxine-pyrimethamine in Bundi Bugyo, western Uganda.Trop Med Int Health. 2004 Apr;9(4):445-50. doi: 10.1111/j.1365-3156.2004.01217.x. Trop Med Int Health. 2004. PMID: 15078262 Clinical Trial.
-
Efficacy of combination therapy with artesunate plus amodiaquine compared to monotherapy with chloroquine, amodiaquine or sulfadoxine-pyrimethamine for treatment of uncomplicated Plasmodium falciparum in Afghanistan.Trop Med Int Health. 2005 Jun;10(6):521-9. doi: 10.1111/j.1365-3156.2005.01429.x. Trop Med Int Health. 2005. PMID: 15941414 Clinical Trial.
Cited by
-
Genotyping of Plasmodium falciparum using antigenic polymorphic markers and to study anti-malarial drug resistance markers in malaria endemic areas of Bangladesh.Malar J. 2012 Nov 22;11:386. doi: 10.1186/1475-2875-11-386. Malar J. 2012. PMID: 23173674 Free PMC article.
-
Resistance to therapies for infection by Plasmodium vivax.Clin Microbiol Rev. 2009 Jul;22(3):508-34. doi: 10.1128/CMR.00008-09. Clin Microbiol Rev. 2009. PMID: 19597012 Free PMC article. Review.
-
Efficacy of fixed-dose combination artesunate-amodiaquine versus artemether-lumefantrine for uncomplicated childhood Plasmodium falciparum malaria in Democratic Republic of Congo: a randomized non-inferiority trial.Malar J. 2012 May 25;11:174. doi: 10.1186/1475-2875-11-174. Malar J. 2012. PMID: 22631564 Free PMC article. Clinical Trial.
-
Statistical methods to derive efficacy estimates of anti-malarials for uncomplicated Plasmodium falciparum malaria: pitfalls and challenges.Malar J. 2017 Oct 26;16(1):430. doi: 10.1186/s12936-017-2074-7. Malar J. 2017. PMID: 29073901 Free PMC article. Review.
-
The rationale and plan for creating a World Antimalarial Resistance Network (WARN).Malar J. 2007 Sep 6;6:118. doi: 10.1186/1475-2875-6-118. Malar J. 2007. PMID: 17822531 Free PMC article.
References
-
- Bachy, C., F. Checchi, and P. Cavailler. 2003. Chloroquine and sulphadoxine-pyrimethamine drug sensitivity study for the treatment of uncomplicated malaria due to Plasmodium falciparum in Mapel, Sudan. Epicentre/MSF, Paris, France.
-
- Basco, L. K., and P. Ringwald. 2000. Molecular epidemiology of malaria in Yaounde, Cameroon. VII. Analysis of recrudescence and reinfection in patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 63:215-221. - PubMed
-
- Bonnet, M. 2004. Efficacité de la sulfadoxine-pyriméthamine, amodiaquine, artesunate plus sulfadoxine-pyriméthamine et artesunate plus amodiaquine pour le traitement du paludisme non-compliqué à Plasmodium falciparum, dans le Centre de Santé de Boende, République Démocratique du Congo. Epicentre/MSF Report, Paris, France.
-
- Checchi, F., S. Balkan, B. T. Vonhm, M. Massaquoi, P. Biberson, P. Eldin de Pecoulas, P. Brasseur, and J.-P. Guthmann. 2002. Efficacy of amodiaquine for uncomplicated Plasmodium falciparum malaria in Harper, Liberia. Trans. R. Soc. Trop. Med. Hyg. 96:670-673. - PubMed
-
- Checchi, F., P. Piola, C. Kosack, E. Ardizzoni, D. Klarkowski, E. Kwezi, G. Priotto, S. Balkan, N. Bakyaita, A. Brockman, and J.-P. Guthmann. 2004. Antimalarial efficacy of sulfadoxine-pyrimethamine, amodiaquine and a combination of chloroquine plus sulfadoxine-pyrimethamine in Bundi Bugyo, western Uganda. Trop. Med. Int. Health 9:445-450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

