Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis
- PMID: 16954319
- PMCID: PMC1635198
- DOI: 10.1128/AAC.00507-06
Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis
Abstract
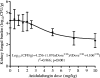
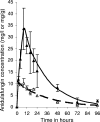
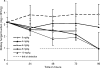
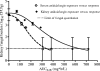
Candidemia is often fatal, especially in patients with persistent neutropenia. New therapies are needed. We performed 24-h pharmacodynamic studies to compare the efficacies of anidulafungin, fluconazole, and amphotericin B in neutropenic mice with disseminated candidiasis caused by one of three strains of Candida glabrata. Anidulafungin produced a maximal fungal kill (E(max)) of 1.4 to 1.9 log(10) CFU/g in kidneys and was not influenced by resistance to either fluconazole or amphotericin B. Fluconazole produced an E(max) of 1.3 log(10) CFU/g in mice infected with fluconazole-susceptible C. glabrata, but the E(max) was 0 for mice infected with a C. glabrata strain that had a fluconazole MIC of >/=32 mg/liter. Amphotericin B achieved an E(max) of 4.2 log(10) CFU/g in mice infected with amphotericin B-susceptible C. glabrata, but the E(max) was 0 for mice infected with a C. glabrata strain with an amphotericin B MIC of 2 mg/liter. In all instances, anidulafungin's maximal microbial kill was superior to that of fluconazole. Next, we performed a 96-h anidulafungin pharmacokinetic-pharmacodynamic study. Anidulafungin exhibited delayed peak concentrations in kidneys compared to those in serum, after which the concentrations declined, with a serum terminal half-life of 21.6 (+/-4.6) h. This was accompanied by a persistent 96-h decrease in the kidney fungal burden after treatment with a single anidulafungin dose of >/=8 mg/kg of body weight. This pharmacokinetic-pharmacodynamic picture of anidulafungin persistence in tissues and the resultant persistent fungal decline should be exploited to improve the efficacy of anidulafungin therapy for candidemia.
Figures
Similar articles
-
Once-weekly micafungin therapy is as effective as daily therapy for disseminated candidiasis in mice with persistent neutropenia.Antimicrob Agents Chemother. 2007 Mar;51(3):968-74. doi: 10.1128/AAC.01337-06. Epub 2006 Dec 28. Antimicrob Agents Chemother. 2007. PMID: 17194830 Free PMC article.
-
Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling.Antimicrob Agents Chemother. 2001 Oct;45(10):2845-55. doi: 10.1128/AAC.45.10.2845-2855.2001. Antimicrob Agents Chemother. 2001. PMID: 11557479 Free PMC article.
-
Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model.Antimicrob Agents Chemother. 2001 Mar;45(3):922-6. doi: 10.1128/AAC.45.3.922-926.2001. Antimicrob Agents Chemother. 2001. PMID: 11181381 Free PMC article.
-
Anidulafungin: a new echinocandin with a novel profile.Clin Ther. 2005 Jun;27(6):657-73. doi: 10.1016/j.clinthera.2005.06.010. Clin Ther. 2005. PMID: 16117974 Review.
-
The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications.Pharmacology. 2006;78(4):161-77. doi: 10.1159/000096348. Epub 2006 Oct 17. Pharmacology. 2006. PMID: 17047411 Review.
Cited by
-
Pharmacodynamics of anidulafungin against clinical Aspergillus fumigatus isolates in a nonneutropenic murine model of disseminated aspergillosis.Antimicrob Agents Chemother. 2013 Jan;57(1):303-8. doi: 10.1128/AAC.01430-12. Epub 2012 Oct 31. Antimicrob Agents Chemother. 2013. PMID: 23114773 Free PMC article.
-
Preclinical Pharmacokinetic/Pharmacodynamic Studies and Clinical Trials in the Drug Development Process of EMA-Approved Antifungal Agents: A Review.Clin Pharmacokinet. 2024 Jan;63(1):13-26. doi: 10.1007/s40262-023-01327-2. Epub 2023 Nov 16. Clin Pharmacokinet. 2024. PMID: 37971649 Free PMC article. Review.
-
PK/PD modeling and simulation of the in vitro activity of the combinations of isavuconazole with echinocandins against Candida auris.CPT Pharmacometrics Syst Pharmacol. 2023 Jun;12(6):770-782. doi: 10.1002/psp4.12949. Epub 2023 Mar 13. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36915233 Free PMC article.
-
Combination of Systemic and Lock-Therapies with Micafungin Eradicate Catheter-Based Biofilms and Infections Caused by Candida albicans and Candida parapsilosis in Neutropenic Rabbit Models.J Fungi (Basel). 2024 Apr 17;10(4):293. doi: 10.3390/jof10040293. J Fungi (Basel). 2024. PMID: 38667964 Free PMC article.
-
In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera.Antimicrob Agents Chemother. 2007 May;51(5):1616-20. doi: 10.1128/AAC.00105-07. Epub 2007 Feb 16. Antimicrob Agents Chemother. 2007. PMID: 17307976 Free PMC article.
References
-
- Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. - PubMed
-
- Arendrup, M. C., K. Fuursted, B. Gahrn-Hansen, I. M. Jensen, J. D. Knudsen, B. Lundgren, H. C. Schonheyder, and M. Tvede. 2005. Seminational surveillance of fungemia in Denmark: notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J. Clin. Microbiol. 43:4434-4440. - PMC - PubMed
-
- Bates, D. W., L. Su, D. T. Yu, G. M. Chertow, D. L. Seger, D. R. Gomes, E. J. Dasbach, and R. Platt. 2001. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin. Infect. Dis. 32:686-693. - PubMed
-
- Bodey, G. P., M. Mardani, H. A. Hanna, M. Boktour, J. Abbas, E. Girgawy, R. Y. Hachem, D. P. Kontoyiannis, and I. I. Raad. 2002. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am. J. Med. 112:380-385. - PubMed
-
- Brieland, J., D. Essig, C. Jackson, D. Frank, D. Loebenberg, F. Menzel, B. Arnold, B. DiDomenico, and R. Hare. 2001. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 69:5046-5055. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

