Identification of yin-yang regulators and a phosphorylation consensus for male germ cell-associated kinase (MAK)-related kinase
- PMID: 16954377
- PMCID: PMC1636783
- DOI: 10.1128/MCB.00816-06
Identification of yin-yang regulators and a phosphorylation consensus for male germ cell-associated kinase (MAK)-related kinase
Abstract
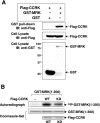
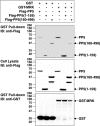
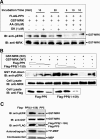
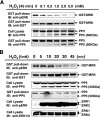
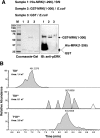
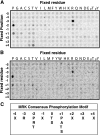
MAK (male germ cell-associated protein kinase) and MRK/ICK (MAK-related kinase/intestinal cell kinase) are human homologs of Ime2p in Saccharomyces cerevisiae and of Mde3 and Pit1 in Schizosaccharomyces pombe and are similar to human cyclin-dependent kinase 2 (CDK2) and extracellular signal-regulated kinase 2 (ERK2). MAK and MRK require dual phosphorylation in a TDY motif catalyzed by an unidentified human threonine kinase and tyrosine autophosphorylation. Herein, we establish that human CDK-related kinase CCRK (cell cycle-related kinase) is an activating T157 kinase for MRK, whereas active CDK7/cyclin H/MAT1 complexes phosphorylate CDK2 but not MRK. Protein phosphatase 5 (PP5) interacts with MRK in a complex and dephosphorylates MRK at T157 in vitro and in situ. Thus, CCRK and PP5 are yin-yang regulators of T157 phosphorylation. To determine a substrate consensus, we screened a combinatorial peptide library with active MRK. MRK preferentially phosphorylates R-P-X-S/T-P sites, with the preference for arginine at position -3 (P-3) being more stringent than for prolines at P-2 and P+1. Using the consensus, we identified a putative phosphorylation site (RPLT(1080)S) for MRK in human Scythe, an antiapoptotic protein that interacts with MRK. MRK phosphorylates Scythe at T1080 in vitro as determined by site-directed mutagenesis and mass spectrometry, supporting the consensus and suggesting Scythe as a physiological substrate for MRK.
Figures






Similar articles
-
Activation of a nuclear Cdc2-related kinase within a mitogen-activated protein kinase-like TDY motif by autophosphorylation and cyclin-dependent protein kinase-activating kinase.Mol Cell Biol. 2005 Jul;25(14):6047-64. doi: 10.1128/MCB.25.14.6047-6064.2005. Mol Cell Biol. 2005. PMID: 15988018 Free PMC article.
-
Reciprocal activation by cyclin-dependent kinases 2 and 7 is directed by substrate specificity determinants outside the T loop.Mol Cell Biol. 2001 Jan;21(1):88-99. doi: 10.1128/MCB.21.1.88-99.2001. Mol Cell Biol. 2001. PMID: 11113184 Free PMC article.
-
HIRA, the human homologue of yeast Hir1p and Hir2p, is a novel cyclin-cdk2 substrate whose expression blocks S-phase progression.Mol Cell Biol. 2001 Mar;21(5):1854-65. doi: 10.1128/MCB.21.5.1854-1865.2001. Mol Cell Biol. 2001. PMID: 11238922 Free PMC article.
-
Dephosphorylation of Cdk2 Thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin.Science. 1995 Oct 6;270(5233):90-3. doi: 10.1126/science.270.5233.90. Science. 1995. PMID: 7569954
-
Control of eukaryotic cell cycle progression by phosphorylation of cyclin-dependent kinases.Cancer J Sci Am. 1998 May;4 Suppl 1:S77-83. Cancer J Sci Am. 1998. PMID: 9619275 Review. No abstract available.
Cited by
-
Transcriptional profiling of endocrine cerebro-osteodysplasia using microarray and next-generation sequencing.PLoS One. 2011;6(9):e25400. doi: 10.1371/journal.pone.0025400. Epub 2011 Sep 27. PLoS One. 2011. PMID: 21980446 Free PMC article.
-
Ssp2 Binding Activates the Smk1 Mitogen-Activated Protein Kinase.Mol Cell Biol. 2017 May 2;37(10):e00607-16. doi: 10.1128/MCB.00607-16. Print 2017 May 15. Mol Cell Biol. 2017. PMID: 28223369 Free PMC article.
-
CCRK depletion inhibits glioblastoma cell proliferation in a cilium-dependent manner.EMBO Rep. 2013 Aug;14(8):741-7. doi: 10.1038/embor.2013.80. Epub 2013 Jun 7. EMBO Rep. 2013. PMID: 23743448 Free PMC article.
-
Kinase mutations in human disease: interpreting genotype-phenotype relationships.Nat Rev Genet. 2010 Jan;11(1):60-74. doi: 10.1038/nrg2707. Nat Rev Genet. 2010. PMID: 20019687 Review.
-
Ciliogenesis associated kinase 1: targets and functions in various organ systems.FEBS Lett. 2019 Nov;593(21):2990-3002. doi: 10.1002/1873-3468.13600. Epub 2019 Sep 20. FEBS Lett. 2019. PMID: 31506943 Free PMC article. Review.
References
-
- Abe, S., T. Yagi, S. Ishiyama, M. Hiroe, F. Marumo, and Y. Ikawa. 1995. Molecular cloning of a novel serine/threonine kinase, MRK, possibly involved in cardiac development. Oncogene 11:2187-2195. - PubMed
-
- Barzilai, A., and K. Yamamoto. 2004. DNA damage responses to oxidative stress. DNA Repair 3:1109-1115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
