Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc
- PMID: 16954387
- PMCID: PMC1636748
- DOI: 10.1128/MCB.01091-06
Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc
Abstract
Recent work with mouse models and human leukemic samples has shown that gain-of-function mutation(s) in Notch1 is a common genetic event in T-cell acute lymphoblastic leukemia (T-ALL). The Notch1 receptor signals through a gamma-secretase-dependent process that releases intracellular Notch1 from the membrane to the nucleus, where it forms part of a transcriptional activator complex. To identify Notch1 target genes in leukemia, we developed mouse T-cell leukemic lines that express intracellular Notch1 in a doxycycline-dependent manner. Using gene expression profiling and chromatin immunoprecipitation, we identified c-myc as a novel, direct, and critical Notch1 target gene in T-cell leukemia. c-myc mRNA levels are increased in primary mouse T-cell tumors that harbor Notch1 mutations, and Notch1 inhibition decreases c-myc mRNA levels and inhibits leukemic cell growth. Retroviral expression of c-myc, like intracellular Notch1, rescues the growth arrest and apoptosis associated with gamma-secretase inhibitor treatment or Notch1 inhibition. Consistent with these findings, retroviral insertional mutagenesis screening of our T-cell leukemia mouse model revealed common insertions in either notch1 or c-myc genes. These studies define the Notch1 molecular signature in mouse T-ALL and importantly provide mechanistic insight as to how Notch1 contributes to human T-ALL.
Figures


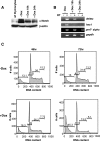
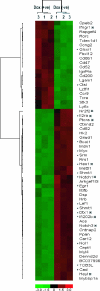
 ; c-myc target genes are indicated by an asterisk.
; c-myc target genes are indicated by an asterisk.

References
-
- Adler, S. H., E. Chiffoleau, L. Xu, N. M. Dalton, J. M. Burg, A. D. Wells, M. S. Wolfe, L. A. Turka, and W. S. Pear. 2003. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J. Immunol. 171:2896-2903. - PubMed
-
- Bain, G., E. C. Maandag, D. J. Izon, D. Amsen, A. M. Kruisbeek, B. C. Weintraub, I. Krop, M. S. Schlissel, A. J. Feeney, and M. van Roon. 1994. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell 79:885-892. - PubMed
-
- Beverly, L. J., and A. J. Capobianco. 2003. Perturbation of Ikaros isoform selection by MLV integration is a cooperative event in Notch(IC)-induced T cell leukemogenesis. Cancer Cell 3:551-564. - PubMed
-
- Beverly, L. J., D. W. Felsher, and A. J. Capobianco. 2005. Suppression of p53 by Notch in lymphomagenesis: implications for initiation and regression. Cancer Res. 65:7159-7168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
