A gradient of epidermal growth factor receptor signaling determines the sensitivity of rbf1 mutant cells to E2F-dependent apoptosis
- PMID: 16954388
- PMCID: PMC1636876
- DOI: 10.1128/MCB.00836-06
A gradient of epidermal growth factor receptor signaling determines the sensitivity of rbf1 mutant cells to E2F-dependent apoptosis
Abstract
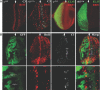
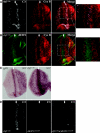
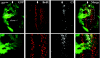
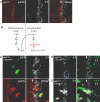
The inactivation of retinoblastoma (Rb) family members sensitizes cells to apoptosis. This cell death affects the development of mutant animals and also provides a critical constraint to the malignant potential of Rb mutant tumor cells. The extent of apoptosis caused by the inactivation of Rb is highly cell type and tissue specific, but the underlying reasons for this variation are poorly understood. Here, we characterize a specific time and place during Drosophila melanogaster development where rbf1 mutant cells are exquisitely sensitive to apoptosis. During the third larval instar, many rbf1 mutant cells undergo E2F-dependent cell death in the morphogenetic furrow. Surprisingly, this pattern of apoptosis is not caused by inappropriate cell cycle progression but instead involves the action of Argos, a secreted protein that negatively regulates Drosophila epidermal growth factor receptor (EGFR [DER]) activity. Apoptosis of rbf1 mutant cells is suppressed by the activation of DER, ras, or raf or by the inactivation of argos, sprouty, or gap1, and inhibition of DER strongly enhances apoptosis in rbf1 mutant discs. We show that RBF1 and a DER/ras/raf signaling pathway cooperate in vivo to suppress E2F-dependent apoptosis and that the loss of RBF1 alters a normal program of cell death that is controlled by Argos and DER. These results demonstrate that a gradient of DER/ras/raf signaling that occurs naturally during development provides the contextual signals that determine when and where the inactivation of rbf1 results in dE2F1-dependent apoptosis.
Figures
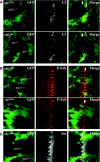
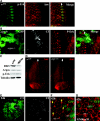
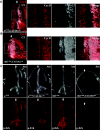
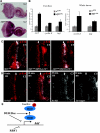
References
-
- Abrams, J. M. 1999. An emerging blueprint for apoptosis in Drosophila. Trends Cell Biol. 9:435-440. - PubMed
-
- Asano, M., J. R. Nevins, and R. P. Wharton. 1996. Ectopic E2F expression induces S phase and apoptosis in Drosophila imaginal discs. Genes Dev. 10:1422-1432. - PubMed
-
- Baker, N. E., and S. Y. Yu. 2001. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell 104:699-708. - PubMed
-
- Baonza, A., T. Casci, and M. Freeman. 2001. A primary role for the epidermal growth factor receptor in ommatidial spacing in the Drosophila eye. Curr. Biol. 11:396-404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
