Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo
- PMID: 16954399
- PMCID: PMC1695516
- DOI: 10.1128/IAI.00721-06
Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo
Abstract
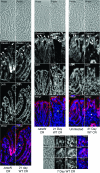
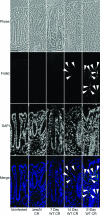
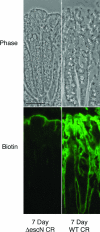
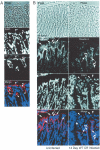
It is widely accepted that tight junctions are altered during infections by attaching and effacing (A/E) pathogens. These disruptions have been demonstrated both in vitro and more recently in vivo. For in vivo experiments, the murine model of A/E infection with Citrobacter rodentium is the animal model of choice. In addition to effects on tight junctions, these bacteria also colonize the colon at high levels, efface colonocyte microvilli, and cause hyperplasia and inflammation. Although we have recently demonstrated that tight junctions are disrupted by C. rodentium, the issue of direct effects of bacteria on epithelial cell junctions versus the indirect effects of inflammation still remains to be clarified. Here, we demonstrate that during the C. rodentium infections, inflammation plays no discernible role in the alteration of tight junctions. The distribution of the tight junction proteins, claudin-1, -3, and -5, are unaffected in inflamed colon, and junctions appear morphologically unaltered when viewed by electron microscopy. Additionally, tracer molecules are not capable of penetrating the inflamed colonic epithelium of infected mice that have cleared the bacteria. Finally, infected colonocytes from mice exposed to C. rodentium for 14 days, which have high levels of bacterial attachment to colonocytes as well as inflammation, have characteristic, altered claudin localization whereas cells adjacent to infected colonocytes retain their normal claudin distribution. We conclude that inflammation plays no discernible role in tight junction alteration during A/E pathogenesis and that tight junction disruption in vivo appears dependent only on the direct intimate attachment of the pathogenic bacteria to the cells.
Figures



References
-
- Barmeyer, C., M. Harren, H. Schmitz, U. Heinzel-Pleines, J. Mankertz, U. Seidler, I. Horak, B. Wiedenmann, M. Fromm, and J. D. Schulzke. 2004. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G244-G252. - PubMed
-
- Chakravortty, D., and K. S. Kumar. 1999. Interaction of lipopolysaccharide with human small intestinal lamina propria fibroblasts favors neutrophil migration and peripheral blood mononuclear cell adhesion by the production of proinflammatory mediators and adhesion molecules. Biochim. Biophys. Acta 1453:261-272. - PubMed
-
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

