A newly identified leptospiral adhesin mediates attachment to laminin
- PMID: 16954400
- PMCID: PMC1695492
- DOI: 10.1128/IAI.00460-06
A newly identified leptospiral adhesin mediates attachment to laminin
Abstract
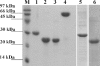
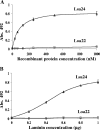
Pathogenic leptospires have the ability to survive and disseminate to multiple organs after penetrating the host. Several pathogens, including spirochetes, have been shown to express surface proteins that interact with the extracellular matrix (ECM). This adhesin-mediated binding process seems to be a crucial step in the colonization of host tissues. This study examined the interaction of putative leptospiral outer membrane proteins with laminin, collagen type I, collagen type IV, cellular fibronectin, and plasma fibronectin. Six predicted coding sequences selected from the Leptospira interrogans serovar Copenhageni genome were cloned, and proteins were expressed, purified by metal affinity chromatography, and characterized by circular dichroism spectroscopy. Their capacity to mediate attachment to ECM components was evaluated by binding assays. We have identified a leptospiral protein encoded by LIC12906, named Lsa24 (leptospiral surface adhesin; 24 kDa) that binds strongly to laminin. Attachment of Lsa24 to laminin was specific, dose dependent, and saturable. Laminin oxidation by sodium metaperiodate reduced the protein-laminin interaction in a concentration-dependent manner, indicating that laminin sugar moieties are crucial for this interaction. Triton X-114-solubilized extract of L. interrogans and phase partitioning showed that Lsa24 was exclusively in the detergent phase, indicating that it is a component of the leptospiral membrane. Moreover, Lsa24 partially inhibited leptospiral adherence to immobilized laminin. This newly identified membrane protein may play a role in mediating adhesion of L. interrogans to the host. To our knowledge, this is the first leptospiral adhesin with laminin-binding properties reported to date.
Figures


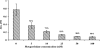



References
-
- Areas, A. P.,M. L. Oliveira, C. R. Ramos, M. E. Sbrogio-Almeida, I. Raw, and P. L. Ho. 2002. Synthesis of cholera toxin B subunit gene: cloning and expression of a functional 6XHis-tagged protein in Escherichia coli. Protein Expr. Purif. 25:481-487. - PubMed
-
- Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. - PubMed
-
- Brown, E. L., B. P. Guo, P. O'Neal, and M. Hook. 1999. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J. Biol. Chem. 274:26272-26278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

