Amino acid residues within enterohemorrhagic Escherichia coli O157:H7 Tir involved in phosphorylation, alpha-actinin recruitment, and Nck-independent pedestal formation
- PMID: 16954405
- PMCID: PMC1695534
- DOI: 10.1128/IAI.00753-06
Amino acid residues within enterohemorrhagic Escherichia coli O157:H7 Tir involved in phosphorylation, alpha-actinin recruitment, and Nck-independent pedestal formation
Abstract
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 and enteropathogenic E. coli (EPEC) adherence to epithelial cells results in the formation of actin pedestals. Pedestal formation requires the bacterial protein Tir, which is inserted into the epithelial cell plasma membrane by the type III secretion system. EPEC and EHEC use different Tir-based mechanisms for pedestal formation, and the EPEC Tir residues required have been well described. In contrast, little is known about the regions of EHEC O157:H7 Tir that are essential for pedestal formation. Additionally, EHEC O157:H7 Tir is serine/threonine phosphorylated, although the residues involved and their role in pedestal formation are not known. In this study, we describe two regions within the carboxy terminus of EHEC O157:H7 Tir that are required for phosphorylation and pedestal formation. Serines 436 and 437 are substrates for protein kinase A phosphorylation, although this is not required to form pedestals. Using a series of internal deletion mutants, we found that amino acids 454 to 463 are required for efficient pedestal formation. Deleting this region resulted in a significant decrease in the recruitment of both filamentous actin and the actin binding protein alpha-actinin. As alpha-actinin binds directly to the EHEC O157:H7 amino terminus, these data suggest that its recruitment is dependent on pedestal formation.
Figures

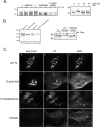


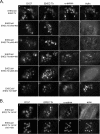
Similar articles
-
Enterohaemorrhagic Escherichia coli Tir requires a C-terminal 12-residue peptide to initiate EspF-mediated actin assembly and harbours N-terminal sequences that influence pedestal length.Cell Microbiol. 2006 Sep;8(9):1488-503. doi: 10.1111/j.1462-5822.2006.00728.x. Cell Microbiol. 2006. PMID: 16922867
-
A carboxy-terminal domain of Tir from enterohemorrhagic Escherichia coli O157:H7 (EHEC O157:H7) required for efficient type III secretion.FEMS Microbiol Lett. 2005 Feb 15;243(2):355-64. doi: 10.1016/j.femsle.2004.12.027. FEMS Microbiol Lett. 2005. PMID: 15686835
-
Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals.Infect Immun. 2001 May;69(5):3315-22. doi: 10.1128/IAI.69.5.3315-3322.2001. Infect Immun. 2001. PMID: 11292754 Free PMC article.
-
Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohemorrhagic E. coli O157:H7.Curr Opin Microbiol. 2003 Feb;6(1):82-90. doi: 10.1016/s1369-5274(03)00005-5. Curr Opin Microbiol. 2003. PMID: 12615225 Review.
-
Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal.Cell Microbiol. 2008 Mar;10(3):549-56. doi: 10.1111/j.1462-5822.2007.01103.x. Epub 2007 Dec 4. Cell Microbiol. 2008. PMID: 18053003 Review.
Cited by
-
Membrane-deforming proteins play distinct roles in actin pedestal biogenesis by enterohemorrhagic Escherichia coli.J Biol Chem. 2012 Jun 8;287(24):20613-24. doi: 10.1074/jbc.M112.363473. Epub 2012 Apr 27. J Biol Chem. 2012. PMID: 22544751 Free PMC article.
-
Modelling of infection by enteropathogenic Escherichia coli strains in lineages 2 and 4 ex vivo and in vivo by using Citrobacter rodentium expressing TccP.Infect Immun. 2009 Apr;77(4):1304-14. doi: 10.1128/IAI.01351-08. Epub 2009 Feb 2. Infect Immun. 2009. PMID: 19188355 Free PMC article.
-
Enterohemorrhagic E. coli requires N-WASP for efficient type III translocation but not for EspFU-mediated actin pedestal formation.PLoS Pathog. 2010 Aug 19;6(8):e1001056. doi: 10.1371/journal.ppat.1001056. PLoS Pathog. 2010. PMID: 20808845 Free PMC article.
-
Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6754-9. doi: 10.1073/pnas.0809131106. Epub 2009 Apr 6. Proc Natl Acad Sci U S A. 2009. PMID: 19366662 Free PMC article.
-
TccP2-mediated subversion of actin dynamics by EPEC 2 - a distinct evolutionary lineage of enteropathogenic Escherichia coli.Microbiology (Reading). 2007 Jun;153(Pt 6):1743-1755. doi: 10.1099/mic.0.2006/004325-0. Microbiology (Reading). 2007. PMID: 17526832 Free PMC article.
References
-
- Allen-Vercoe, E., M. C. Toh, B. Waddell, H. Ho, and R. DeVinney. 2005. A carboxy-terminal domain of Tir from enterohemorrhagic Escherichia coli O157:H7 (EHEC O157:H7) required for efficient type III secretion. FEMS Microbiol. Lett. 243:355-364. - PubMed
-
- Allen-Vercoe, E., B. Waddell, S. Livingstone, J. Deans, and R. Devinney. 2006. Enteropathogenic Escherichia coli Tir translocation and pedestal formation requires membrane cholesterol in the absence of bundle-forming pili. Cell. Microbiol. 8:613-624. - PubMed
-
- Anderson, M. P., R. J. Gregory, S. Thompson, D. W. Souza, S. Paul, R. C. Mulligan, A. E. Smith, and M. J. Welsh. 1991. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253:202-205. - PubMed
-
- Campellone, K., M. Brady, J. Alamares, D. Rowe, B. Skehan, D. Tipper, and J. Leong. 2006. Enterohemorrhagic Escherichia coli Tir requires a C-terminal 12-residue peptide to initiate EspFU-mediated actin assembly and harbours N-terminal sequences that influence pedestal length. Cell. Microbiol. 8:1488-1503. - PubMed
-
- Campellone, K., A. Giese, D. Tipper, and J. Leong. 2002. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol. Microbiol. 43:1227-1241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

