Synthetic oligosaccharide stimulates and stabilizes angiogenesis: structure-function relationships and potential mechanisms
- PMID: 16954815
- PMCID: PMC4140568
- DOI: 10.1097/01.fjc.0000238591.90062.62
Synthetic oligosaccharide stimulates and stabilizes angiogenesis: structure-function relationships and potential mechanisms
Erratum in
-
Synthetic Oligosaccharide Stimulates and Stabilizes Angiogenesis: Structure-Function Relationships and Potential Mechanisms: Erratum.J Cardiovasc Pharmacol. 2022 Oct 1;80(4):629. doi: 10.1097/FJC.0000000000001371. J Cardiovasc Pharmacol. 2022. PMID: 36201458 No abstract available.
Abstract
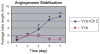
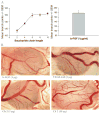
To determine the proangiogenesis effect of series of saccharides and a synthetic oligosaccharide and potential mechanisms, an in vitro 3-dimensional endothelial cell sprouting (3D-ECS) assay and the chick chorioallantoic membrane (CAM) model were used. We demonstrated that a sulfated oligosaccharide significantly promotes the endothelial capillary network initiated by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (b-FGF). Furthermore, although the capillary network initiated by VEGF and b-FGF lasts no more than 7 days, addition of a sulfated oligosaccharide significantly amplifies angiogenesis and stabilizes the capillary network of new blood vessels. In the CAM model, sulfated oligosaccharide also stimulated angiogenesis. In both the CAM and the 3D-ECS assay, structure-function studies reveal that increased saccharide chain length up to the hexa- to decasaccharide show optimal proangiogenesis efficacy. In addition, the sulfation and molecular shape (branched vs linear) of oligosaccharide are important for sustained proangiogenesis efficacy. Data indicate that chemically defined synthetic oligosaccharides can play an important role in regulation of capillary structure and stability, which may contribute to future advances in therapeutic angiogenesis. The proangiogenesis efficacy of an oligosaccharide is mediated via integrin alphavbeta3 and involves mitogen-activated protein kinase signaling mechanisms.
Figures




Similar articles
-
Proangiogenesis action of the thyroid hormone analog 3,5-diiodothyropropionic acid (DITPA) is initiated at the cell surface and is integrin mediated.Endocrinology. 2006 Apr;147(4):1602-7. doi: 10.1210/en.2005-1390. Epub 2005 Dec 29. Endocrinology. 2006. PMID: 16384862
-
Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor.Angiogenesis. 2008;11(2):183-90. doi: 10.1007/s10456-007-9088-7. Epub 2007 Dec 13. Angiogenesis. 2008. PMID: 18080776
-
Evaluation of angiogenic activities of hyaluronan oligosaccharides of defined minimum size.Life Sci. 2009 Oct 7;85(15-16):573-7. doi: 10.1016/j.lfs.2009.08.010. Epub 2009 Aug 29. Life Sci. 2009. PMID: 19720068
-
Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo.Anat Rec. 2001 Dec 1;264(4):317-24. doi: 10.1002/ar.10021. Anat Rec. 2001. PMID: 11745087 Review.
-
Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis.Int Rev Cell Mol Biol. 2008;270:181-224. doi: 10.1016/S1937-6448(08)01405-6. Int Rev Cell Mol Biol. 2008. PMID: 19081537 Review.
Cited by
-
Inhibitors of slit protein interactions with the heparan sulphate proteoglycan glypican-1: potential agents for the treatment of spinal cord injury.Clin Exp Pharmacol Physiol. 2010 Apr;37(4):417-21. doi: 10.1111/j.1440-1681.2009.05318.x. Epub 2009 Oct 16. Clin Exp Pharmacol Physiol. 2010. PMID: 19843094 Free PMC article.
-
Glycosyl ortho-(1-phenylvinyl)benzoates versatile glycosyl donors for highly efficient synthesis of both O-glycosides and nucleosides.Nat Commun. 2020 Jan 21;11(1):405. doi: 10.1038/s41467-020-14295-z. Nat Commun. 2020. PMID: 31964883 Free PMC article.
-
Suppression of pancreatic cancer by sulfated non-anticoagulant low molecular weight heparin.Cancer Lett. 2014 Aug 1;350(1-2):25-33. doi: 10.1016/j.canlet.2014.04.016. Epub 2014 Apr 24. Cancer Lett. 2014. PMID: 24769074 Free PMC article.
-
An orthogonal and reactivity-based one-pot glycosylation strategy for both glycan and nucleoside synthesis: access to TMG-chitotriomycin, lipochitooligosaccharides and capuramycin.Chem Sci. 2021 Feb 23;12(14):5143-5151. doi: 10.1039/d0sc06815b. Chem Sci. 2021. PMID: 34163751 Free PMC article.
-
Effect of heparan sulfate and gold nanoparticles on muscle development during embryogenesis.Int J Nanomedicine. 2011;6:3163-72. doi: 10.2147/IJN.S26070. Epub 2011 Dec 6. Int J Nanomedicine. 2011. PMID: 22238506 Free PMC article.
References
-
- Carmeliet P, Collen D. Molecular basis of angiogenesis: role of VEGF and VE-cadherin. Ann N Y Acad Sci. 2000;902:249–262. - PubMed
-
- Tomanek RJ, Schatteman GC. Angiogenesis: new insights and therapeutic potential. Anat Rec. 2000;261:126–135. - PubMed
-
- Mousa SA. Angiogenesis promoters and inhibitors: potential therapeutic implications. Mol Med Today. 1996;2:140–142. - PubMed
-
- Hudlicka O. Factors involved in capillary growth in the heart. Mol Cell Biochem. 1995;147:57–68. - PubMed
-
- Folkman J, Weisz PB, Joullie MM, et al. Control of angiogenesis with synthetic heparin substitutes. Science. 1989;243:1490–1493. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

