Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells
- PMID: 16955077
- PMCID: PMC2396349
- DOI: 10.1038/sj.jcbfm.9600395
Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells
Abstract
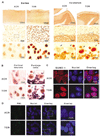
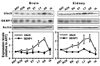
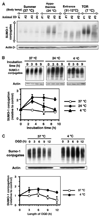
Hibernation torpor provides an excellent natural model of tolerance to profound reductions in blood flow to the brain and other organs. Here, we report that during torpor of 13-lined ground squirrels, massive SUMOylation occurs in the brain, liver, and kidney. The level of small ubiquitin-related modifier (SUMO) conjugation coincides with the expression level of Ubc9, the SUMO specific E2-conjugating enzyme. Hypothermia alone also increased SUMO conjugation, but not as markedly as hibernation torpor. Increased SUMO conjugation (induced by Ubc9 overexpression, ischemic preconditioning (PC)+/-hypothermia) was necessary and sufficient for tolerance of SHSY5Y neuroblastoma cells to oxygen/glucose deprivation (OGD) ('in vitro ischemia'); decreased SUMO conjugation (induced by a dominant-negative Ubc9) severely reduced tolerance to OGD in these cells. These data indicate that post-translational modification of proteins by SUMOylation is a prominent feature of hibernation torpor and is critical for cytoprotection by ischemic PC+/-hypothermia in SHSY5Y cells subjected to OGD.
Figures



References
-
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. - PubMed
-
- Chen Y, Matsushita M, Nairn AC, Damuni Z, Cai D, Frerichs KU, Hallenbeck JM. Mechanisms for increased levels of phosphorylation of elongation factor-2 during hibernation in ground squirrels. Biochemistry. 2001;40:11565–11570. - PubMed
-
- De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. - PubMed
-
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

