Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury
- PMID: 16955137
- PMCID: PMC1555637
- DOI: 10.1172/JCI27154
Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury
Abstract
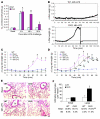
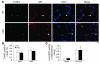
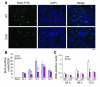
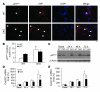
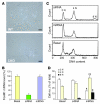
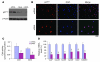
Recovery of endothelial integrity after vascular injury is vital for endothelial barrier function and vascular homeostasis. However, little is known about the molecular mechanisms of endothelial barrier repair following injury. To investigate the functional role of forkhead box M1 (FoxM1) in the mechanism of endothelial repair, we generated endothelial cell-restricted FoxM1-deficient mice (FoxM1 CKO mice). These mutant mice were viable and exhibited no overt phenotype. However, in response to the inflammatory mediator LPS, FoxM1 CKO mice displayed significantly protracted increase in lung vascular permeability and markedly increased mortality. Following LPS-induced vascular injury, FoxM1 CKO lungs demonstrated impaired cell proliferation in association with sustained expression of p27(Kip1) and decreased expression of cyclin B1 and Cdc25C. Endothelial cells isolated from FoxM1 CKO lungs failed to proliferate, and siRNA-mediated suppression of FoxM1 expression in human endothelial cells resulted in defective cell cycle progression. Deletion of FoxM1 in endothelial cells induced decreased expression of cyclins, Cdc2, and Cdc25C, increased p27(Kip1) expression, and decreased Cdk activities. Thus, FoxM1 plays a critical role in the mechanism of the restoration of endothelial barrier function following vascular injury. These data suggest that impairment in FoxM1 activation may be an important determinant of the persistent vascular barrier leakiness and edema formation associated with inflammatory diseases.
Figures
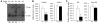
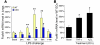


Comment in
-
Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury.J Clin Invest. 2006 Sep;116(9):2316-9. doi: 10.1172/JCI29637. J Clin Invest. 2006. PMID: 16955131 Free PMC article.
References
-
- Cines D.B., et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
-
- Ross R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. - PubMed
-
- Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. - PubMed
-
- Austin G.E., Ratliff N.B., Hollman J., Tabei S., Phillips D.F. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J. Am. Coll. Cardiol. 1985;6:369–375. - PubMed
-
- Schwartz R.S., Holmes D.R., Topol E.J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J. Am. Coll. Cardiol. 1992;20:1284–1293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

