Severe facial clefting in Insig-deficient mouse embryos caused by sterol accumulation and reversed by lovastatin
- PMID: 16955138
- PMCID: PMC1555642
- DOI: 10.1172/JCI28988
Severe facial clefting in Insig-deficient mouse embryos caused by sterol accumulation and reversed by lovastatin
Abstract
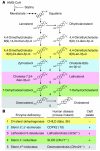
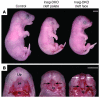
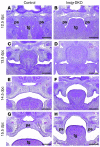
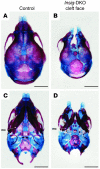
Insig-1 and Insig-2 are regulatory proteins that restrict the cholesterol biosynthetic pathway by preventing proteolytic activation of SREBPs and by enhancing degradation of HMG-CoA reductase. Here, we created Insig-double-knockout (Insig-DKO) mice that are homozygous for null mutations in Insig-1 and Insig-2. After 18.5 days of development, 96% of Insig-DKO embryos had defects in midline facial development, ranging from cleft palate (52%) to complete cleft face (44%). Middle and inner ear structures were abnormal, but teeth and skeletons were normal. The animals were lethargic and runted; they died within 1 day of birth. The livers and heads of Insig-DKO embryos overproduced sterols, causing a marked buildup of sterol intermediates. Treatment of pregnant mice with the HMG-CoA reductase inhibitor lovastatin reduced sterol synthesis in Insig-DKO embryos and reduced the pre-cholesterol intermediates. This treatment ameliorated the clefting syndrome so that 54% of Insig-DKO mice had normal faces, and only 7% had cleft faces. We conclude that buildup of pre-cholesterol sterol intermediates interferes with midline fusion of facial structures in mice. These findings have implications for the pathogenesis of the cleft palate component of Smith-Lemli-Opitz syndrome and other human malformation syndromes in which mutations in enzymes catalyzing steps in cholesterol biosynthesis produce a buildup of sterol intermediates.
Figures


Comment in
-
Cholesterol precursors and facial clefting.J Clin Invest. 2006 Sep;116(9):2322-5. doi: 10.1172/JCI29872. J Clin Invest. 2006. PMID: 16955133 Free PMC article.
References
-
- Kelley R.I., Herman G.E. Inborn errors of sterol biosynthesis. Annu. Rev. Genomics Hum. Genet. 2001;2:299–341. - PubMed
-
- Nwokoro N.A., Wassif C.A., Porter F.D. Genetic disorders of cholesterol biosynthesis in mice and humans. Mol. Genet. Metab. 2001;74:105–119. - PubMed
-
- Porter F.D. Human malformation syndromes due to inborn errors of cholesterol synthesis. . Curr. Opin. Pediatr. 2003;15:607–613. - PubMed
-
- Herman G.E. Disorders of cholesterol biosynthesis: prototypic metabolic malformation syndromes. . Hum. Mol. Genet. 2003;12:R75–R88. - PubMed
-
- Liu X.Y., et al. The gene mutated in bare patches and striated mice encodes a novel 3β-hydroxysteroid dehydrogenase. Nat. Genet. 1999;22:182–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

