CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes
- PMID: 16955139
- PMCID: PMC1555650
- DOI: 10.1172/JCI28796
CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes
Abstract
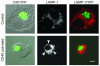
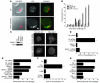
Many intracellular pathogens, including Toxoplasma gondii, survive within macrophages by residing in vacuoles that avoid fusion with lysosomes. It is important to determine whether cell-mediated immunity can trigger macrophage antimicrobial activity by rerouting these vacuoles to lysosomes. We report that CD40 stimulation of human and mouse macrophages infected with T. gondii resulted in fusion of parasitophorous vacuoles and late endosomes/lysosomes. Vacuole/lysosome fusion took place even when CD40 was ligated after the formation of parasitophorous vacuoles. Genetic and pharmacological approaches that impaired phosphoinositide-3-class 3 (PIK3C3), Rab7, vacuolar ATPase, and lysosomal enzymes revealed that vacuole/lysosome fusion mediated antimicrobial activity induced by CD40. Ligation of CD40 caused colocalization of parasitophorous vacuoles and LC3, a marker of autophagy, which is a process that controls lysosomal degradation. Vacuole/lysosome fusion and antimicrobial activity were shown to be dependent on autophagy. Thus, cell-mediated immunity through CD40 stimulation can reroute an intracellular pathogen to the lysosomal compartment, resulting in macrophage antimicrobial activity.
Figures

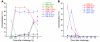


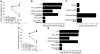

References
-
- Sinai A.P., Joiner K.A. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Ann. Rev. Microbiol. 1997;51:415–462. - PubMed
-
- Meresse S., et al. Controlling the maturation of pathogen-containing vacuoles: a matter of life or death. Nat. Cell Biol. 1999;1:E183–E188. - PubMed
-
- Amer A.O., Swanson M.S. A phagosome of one’s own: a microbial guide to life in the macrophage. . Curr. Opin. Microbiol. 2002;5:56–61. - PubMed
-
- Coppens I., et al. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. . Cell. 2006;125:261–274. - PubMed
-
- Mordue D.G., Sibley L.D. Intracellular fate of vacuoles containingToxoplasma gondii is determined at the time of formation and depends on the mechanisms of entry. . J. Immunol. 1997;159:4452–4459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

