Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice
- PMID: 16955141
- PMCID: PMC1555670
- DOI: 10.1172/JCI28334
Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice
Abstract
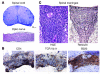
Multiple sclerosis (MS) is a clinically and pathologically heterogeneous inflammatory/demyelinating disease of the CNS. In the MS variant Devic disease, lesions are predominantly found in the optic nerves and spinal cord but not the brain. The immunological bases of the different forms of MS are unknown. We previously generated myelin oligodendrocyte glycoprotein-specific (MOG-specific) TCR transgenic mice (TCRMOG mice; also referred to as 2D2 mice) and reported that a large proportion of these mice develop spontaneous isolated optic neuritis. We have now crossed the TCRMOG mice with MOG-specific Ig heavy-chain knock-in mice (IgHMOG mice; also referred to as Th mice), in which one-third of the B cells are specific for MOG. In these mice, MOG-specific B cells are very efficient in presenting MOG to the transgenic T cells and undergo class switching to IgG1 in the presence of the transgenic T cells. Sixty percent of TCRMOG x IgHMOG mice spontaneously developed a severe form of experimental autoimmune encephalomyelitis (EAE). Histological examination of the CNS revealed a selective distribution of meningeal and parenchymal inflammatory lesions in the spinal cord and optic nerves. Thus, CNS antigen-specific T and B cells cooperate to induce a distinct clinicopathologic EAE pattern that closely replicates human Devic disease.
Figures



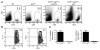
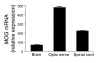
Comment in
-
A mighty mouse: building a better model of multiple sclerosis.J Clin Invest. 2006 Sep;116(9):2313-6. doi: 10.1172/JCI29834. J Clin Invest. 2006. PMID: 16955130 Free PMC article.
References
-
- Lassmann, H. 1998. Pathology of multiple sclerosis. InMcAlpine’s multiple sclerosis. A. Compston, et al., editors. Churchill Livingstone. Hong Kong, People’s Republic of China. 323–358.
-
- Ghezzi A., et al. Long-term follow-up of isolated optic neuritis: the risk of developing multiple sclerosis, its outcome, and the prognostic role of paraclinical tests. J. Neurol. 1999;246:770–775. - PubMed
-
- Soderstrom M. Optic neuritis and multiple sclerosis. Acta Ophthalmol. Scand. 2001;79:223–227. - PubMed
-
- Misu T., et al. Pure optic-spinal form of multiple sclerosis in Japan. Brain. 2002;125:2460–2468. - PubMed
-
- Wingerchuk D.M., Weinshenker B.G. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology. 2003;60:848–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS030843/NS/NINDS NIH HHS/United States
- NS38037/NS/NINDS NIH HHS/United States
- R01 NS045937/NS/NINDS NIH HHS/United States
- NS30843/NS/NINDS NIH HHS/United States
- R01 AI044880/AI/NIAID NIH HHS/United States
- R37 NS030843/NS/NINDS NIH HHS/United States
- NS45937/NS/NINDS NIH HHS/United States
- R56 AI044880/AI/NIAID NIH HHS/United States
- RG2571/RG/CSR NIH HHS/United States
- NS046414/NS/NINDS NIH HHS/United States
- R01 NS046414/NS/NINDS NIH HHS/United States
- R29 NS030843/NS/NINDS NIH HHS/United States
- P01 NS038037/NS/NINDS NIH HHS/United States
- AI44880/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

