Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus
- PMID: 16956943
- PMCID: PMC1642181
- DOI: 10.1128/JVI.01056-06
Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus
Abstract
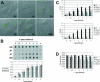
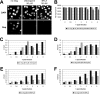
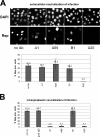
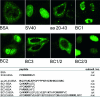
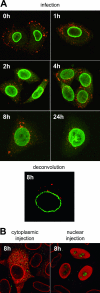
Common features of parvovirus capsids are open pores at the fivefold symmetry axes that traverse the virion shell. Upon limited heat treatment in vitro, the pores can function as portals to externalize VP1/VP2 protein N-terminal sequences which harbor infection-relevant functional domains, such as a phospholipase A(2) catalytic domain. Here we show that adeno-associated virus type 2 (AAV2) also exposes its VP1/VP2 N termini in vivo during infection, presumably in the endosomal compartment. This conformational change is influenced by treatment with lysosomotropic reagents. While incubation of cells with bafilomycin A1 reduced exposure of VP1/VP2 N termini, incubation with chloroquine stimulated externalization transiently. N-terminally located basic amino acid clusters with nuclear localization activity also become exposed in this process and are accessible on the virus capsid when it enters the cytoplasm. This is an obligatory step in AAV2 infection. However, a direct role of these sequences in nuclear translocation of viral capsids could not be determined by microinjection of wild-type or mutant viruses. This suggests that further modifications of the capsid have to take place in a precytoplasmic entry step that prepares the virus for nuclear entry. Microinjection of several capsid-specific antibodies into the cell nucleus blocked AAV2 infection completely, supporting the conclusion that AAV2 capsids bring the infectious genome into the nucleus.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

