Posttranslational modification and cell type-specific degradation of varicella-zoster virus ORF29p
- PMID: 16956951
- PMCID: PMC1641786
- DOI: 10.1128/JVI.00966-06
Posttranslational modification and cell type-specific degradation of varicella-zoster virus ORF29p
Abstract
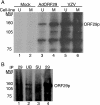
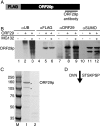
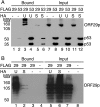
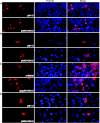
The ORF29 gene of varicella-zoster virus encodes a single-stranded DNA binding protein that is predominantly nuclear during lytic infection but appears to be restricted to the cytoplasm of latently infected neurons. Following reactivation, ORF29p accumulates in the nuclei of neurons, suggesting that its confinement to the cytosol may be critical for maintaining quiescence. When autonomously expressed, ORF29p accumulates in the nuclei of fibroblasts and the cytoplasm of cells (guinea pig enteric neurons) and cell lines (U373MG) of neuronal origin. Inhibition of the 26S proteasome redirects the accumulation of ORF29p to the nucleus in cells of neuronal origin. Here, we show that ORF29p is ubiquitinated and sumoylated in 293T cells and subsequently degraded from the N terminus. Ubiquitinated ORF29p accumulates in both the nuclei and the cytoplasm of fibroblasts, but degradation products are seen primarily in the cytoplasm. Modification and degradation of ORF29p occurs in 293T, U373MG, and MeWo cells. Therefore, these processes are ubiquitous; however, the robustness of the degradation process is cell type specific. The proteasome-mediated mechanism of nuclear exclusion in U373MG cells is an active process that is not specific for the endogenous ORF29p nuclear localization signal but can be saturated by protein stabilization or overexpression, which leads to nuclear accumulation of ORF29p. The evidence for ORF29p ubiquitination and previous data regarding the effect of proteasome inhibitors on the abundance and distribution of ORF29p implicate the 26S proteasome in influencing the protein's cell type-specific localization.
Figures


Similar articles
-
The cellular localization pattern of Varicella-Zoster virus ORF29p is influenced by proteasome-mediated degradation.J Virol. 2006 Feb;80(3):1497-512. doi: 10.1128/JVI.80.3.1497-1512.2006. J Virol. 2006. PMID: 16415026 Free PMC article.
-
Dissection of a novel nuclear localization signal in open reading frame 29 of varicella-zoster virus.J Virol. 2005 Oct;79(20):13070-81. doi: 10.1128/JVI.79.20.13070-13081.2005. J Virol. 2005. PMID: 16189009 Free PMC article.
-
Nuclear import of the varicella-zoster virus latency-associated protein ORF63 in primary neurons requires expression of the lytic protein ORF61 and occurs in a proteasome-dependent manner.J Virol. 2008 Sep;82(17):8673-86. doi: 10.1128/JVI.00685-08. Epub 2008 Jun 18. J Virol. 2008. PMID: 18562514 Free PMC article.
-
Pleiotropic roles of the ubiquitin-proteasome system during viral propagation.Life Sci. 2018 Aug 15;207:350-354. doi: 10.1016/j.lfs.2018.06.014. Epub 2018 Jun 18. Life Sci. 2018. PMID: 29913185 Free PMC article. Review.
-
Regulation and function of SUMO modification.J Biol Chem. 2004 Dec 24;279(52):53899-902. doi: 10.1074/jbc.R400021200. Epub 2004 Sep 24. J Biol Chem. 2004. PMID: 15448161 Review. No abstract available.
Cited by
-
Herpes simplex virus and varicella zoster virus, the house guests who never leave.Herpesviridae. 2012 Jun 12;3(1):5. doi: 10.1186/2042-4280-3-5. Herpesviridae. 2012. PMID: 22691604 Free PMC article.
-
Sumoylation at the host-pathogen interface.Biomolecules. 2012 Apr 5;2(2):203-27. doi: 10.3390/biom2020203. Biomolecules. 2012. PMID: 23795346 Free PMC article.
-
The RING finger domain of Varicella-Zoster virus ORF61p has E3 ubiquitin ligase activity that is essential for efficient autoubiquitination and dispersion of Sp100-containing nuclear bodies.J Virol. 2010 Jul;84(13):6861-5. doi: 10.1128/JVI.00335-10. Epub 2010 Apr 14. J Virol. 2010. PMID: 20392849 Free PMC article.
-
Viral Interplay with the Host Sumoylation System.Adv Exp Med Biol. 2017;963:359-388. doi: 10.1007/978-3-319-50044-7_21. Adv Exp Med Biol. 2017. PMID: 28197923 Free PMC article. Review.
References
-
- Chen, J. J., A. A. Gershon, Z. S. Li, O. Lungu, and M. D. Gershon. 2003. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J. Med. Virol. 70(Suppl. 1):S71-S78. - PubMed
-
- Croen, K. D., and S. E. Straus. 1991. Varicella-zoster virus latency. Annu. Rev. Microbiol. 45:265-282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

