Detection of Brugia parasite DNA in human blood by real-time PCR
- PMID: 16957038
- PMCID: PMC1698366
- DOI: 10.1128/JCM.00969-06
Detection of Brugia parasite DNA in human blood by real-time PCR
Abstract
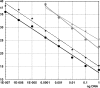
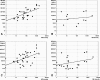
Brugian filariasis (caused by the nematodes Brugia malayi and B. timori) is an important cause of disability in Southeast Asia. Improved diagnostic tests are needed for filariasis elimination programs (to identify areas of endemicity and to monitor progress) and for diagnosis of the disease in infected individuals. We have developed and evaluated two real-time PCR assays for detecting Brugia DNA in human blood and compared the results of these assays to those of "gold standard" assays. One assay uses a TaqMan probe (TaqM) to amplifiy a 320-bp "HhaI repeat" DNA sequence. The other assay uses a minor groove binding probe (MGB) and modified nucleotides in primers (Eclipse MGB) to amplify a 120-bp fragment of the HhaI repeat. This assay detects 22 copies of the target sequence, and it is more sensitive than the TaqM assay. Both assays were evaluated with human blood samples from two different areas of endemicity. The MGB assay was as sensitive as membrane filtration and microscopy for the detection of B. malayi infection in 57 blood samples recovered at night from patients in Sulawesi, Indonesia. The MGB assay also detected parasite DNA in 17 of 31 (55%) of microfilaria-negative day blood samples from these subjects. This test was more sensitive than the conventional and the TaqM PCRs (and was almost as sensitive as night blood membrane filtration) for the detection of infection in 52 blood samples recovered at night from individuals in an area of B. timori endemicity on Alor Island, Indonesia, where microfilaria-positive individuals had low densities after mass treatment. Thus, the Eclipse MGB real-time PCR assay is a sensitive means of detecting Brugia parasite DNA in human blood.
Figures
Similar articles
-
PCR-based detection and identification of the filarial parasite Brugia timori from Alor Island, Indonesia.Ann Trop Med Parasitol. 2002 Dec;96(8):809-21. doi: 10.1179/000349802125002239. Ann Trop Med Parasitol. 2002. PMID: 12625936
-
Detection of DNA of nocturnally periodic Brugia malayi in night and day blood samples by a polymerase chain reaction-ELISA-based method using an internal control DNA.Am J Trop Med Hyg. 2000 Feb;62(2):291-6. doi: 10.4269/ajtmh.2000.62.291. Am J Trop Med Hyg. 2000. PMID: 10813487
-
A polymerase chain reaction assay for the detection of Brugia malayi in blood.Am J Trop Med Hyg. 1994 Sep;51(3):314-21. doi: 10.4269/ajtmh.1994.51.314. Am J Trop Med Hyg. 1994. PMID: 7943550
-
Lymphatic filariasis and Brugia timori: prospects for elimination.Trends Parasitol. 2004 Aug;20(8):351-5. doi: 10.1016/j.pt.2004.06.001. Trends Parasitol. 2004. PMID: 15246315 Review.
-
Recent advances in the application of molecular biology in filariasis.Southeast Asian J Trop Med Public Health. 1993;24 Suppl 2:55-63. Southeast Asian J Trop Med Public Health. 1993. PMID: 7973949 Review.
Cited by
-
Real-time PCR detection of the HhaI tandem DNA repeat in pre- and post-patent Brugia malayi Infections: a study in Indonesian transmigrants.Parasit Vectors. 2014 Mar 31;7:146. doi: 10.1186/1756-3305-7-146. Parasit Vectors. 2014. PMID: 24685183 Free PMC article.
-
A Novel Xenomonitoring Technique Using Mosquito Excreta/Feces for the Detection of Filarial Parasites and Malaria.PLoS Negl Trop Dis. 2016 Apr 20;10(4):e0004641. doi: 10.1371/journal.pntd.0004641. eCollection 2016 Apr. PLoS Negl Trop Dis. 2016. PMID: 27096156 Free PMC article.
-
Microfilaria Positification Test Using Real-Time PCR Technique with HRM (High-Resolution Melting).Acta Parasitol. 2022 Mar;67(1):496-503. doi: 10.1007/s11686-021-00412-5. Epub 2021 Jun 16. Acta Parasitol. 2022. PMID: 34137011
-
Diagnostic Identification and Differentiation of Microfilariae.J Clin Microbiol. 2019 Sep 24;57(10):e00706-19. doi: 10.1128/JCM.00706-19. Print 2019 Oct. J Clin Microbiol. 2019. PMID: 31340993 Free PMC article. Review.
-
A Case for Using Genomics and a Bioinformatics Pipeline to Develop Sensitive and Species-Specific PCR-Based Diagnostics for Soil-Transmitted Helminths.Front Genet. 2019 Sep 23;10:883. doi: 10.3389/fgene.2019.00883. eCollection 2019. Front Genet. 2019. PMID: 31608116 Free PMC article. Review.
References
-
- Bell, A. S., and L. C. Ranford-Cartwright. 2002. Real-time quantitative PCR in parasitology. Trends Parasitol. 18:337-342. - PubMed
-
- Bockarie, M. J., P. Fischer, S. A. Williams, P. A. Zimmerman, L. Griffin, M. P. Alpers, and J. W. Kazura. 2000. Application of a polymerase chain reaction-ELISA to detect Wuchereria bancrofti in pools of wild-caught Anopheles punctulatus in a filariasis control area in Papua New Guinea. Am. J. Trop. Med. Hyg. 62:363-367. - PubMed
-
- Fischer, P., D. Boakye, and J. Hamburger. 2003. Polymerase chain reaction-based detection of lymphatic filariasis. Med. Microbiol. Immunol. (Berlin) 192:3-7. - PubMed
-
- Fischer, P., X. Liu, M. Lizotte-Waniewski, I. H. Kamal, R. M. Ramzy, and S. A. Williams. 1999. Development of a quantitative, competitive polymerase chain reaction-enzyme-linked immunosorbent assay for the detection of Wuchereria bancrofti DNA. Parasitol. Res. 85:176-183. - PubMed
-
- Fischer, P., T. Supali, H. Wibowo, I. Bonow, and S. A. Williams. 2000. Detection of DNA of nocturnally periodic Brugia malayi in night and day blood samples by a polymerase chain reaction-ELISA-based method using an internal control DNA. Am. J. Trop. Med. Hyg. 62:291-296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

