Transformation of the antibacterial agent norfloxacin by environmental mycobacteria
- PMID: 16957195
- PMCID: PMC1563677
- DOI: 10.1128/AEM.03032-05
Transformation of the antibacterial agent norfloxacin by environmental mycobacteria
Abstract
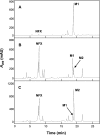
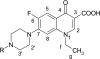
Because fluoroquinolone antimicrobial agents may be released into the environment, the potential for environmental bacteria to biotransform these drugs was investigated. Eight Mycobacterium sp. cultures in a sorbitol-yeast extract medium were dosed with 100 microg ml(-1) of norfloxacin and incubated for 7 days. The MICs of norfloxacin for these strains, tested by an agar dilution method, were 1.6 to 25 microg ml(-1). Cultures were extracted with ethyl acetate, and potential metabolites in the extracts were purified by high-performance liquid chromatography. The metabolites were identified using mass spectrometry and nuclear magnetic resonance spectroscopy. N-Acetylnorfloxacin (5 to 50% of the total absorbance at 280 nm) was produced by the eight Mycobacterium strains. N-Nitrosonorfloxacin (5 to 30% of the total absorbance) was also produced by Mycobacterium sp. strain PYR100 and Mycobacterium gilvum PYR-GCK. The MICs of N-nitrosonorfloxacin and N-acetylnorfloxacin were 2- to 38- and 4- to 1,000-fold higher, respectively, than those of norfloxacin for several different bacteria, including the two strains that produced both metabolites. Although N-nitrosonorfloxacin had less antibacterial activity, nitrosamines are potentially carcinogenic. The biotransformation of fluoroquinolones by mycobacteria may serve as a resistance mechanism.
Figures
References
-
- Alexander, M. 1999. Biodegradation and bioremediation, 2nd ed. Academic Press, San Diego, Calif.
-
- Bhaumik, A. 1997. Effect of norfloxacin treatment in acute enteritis in dogs. Ind. Vet. J. 74:246-247.
-
- Blau, K. 1993. Acylation, p. 31-50. In K. Blau and J. M. Halket (ed.), Handbook of derivatives for chromatography, 2nd ed. John Wiley & Sons, Chichester, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

