Identification, typing, and insecticidal activity of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of the xpt toxin loci
- PMID: 16957209
- PMCID: PMC1563616
- DOI: 10.1128/AEM.00217-06
Identification, typing, and insecticidal activity of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of the xpt toxin loci
Abstract
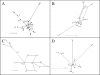
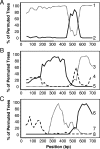
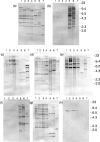
Xenorhabdus strains from entomopathogenic nematodes isolated from United Kingdom soils by using the insect bait entrapment method were characterized by partial sequencing of the 16S rRNA gene, four housekeeping genes (asd, ompR, recA, and serC) and the flagellin gene (fliC). Most strains (191/197) were found to have genes with greatest similarity to those of Xenorhabdus bovienii, and the remaining six strains had genes most similar to those of Xenorhabdus nematophila. Generally, 16S rRNA sequences and the sequence types based on housekeeping genes were in agreement, with a few notable exceptions. Statistical analysis implied that recombination had occurred at the serC locus and that moderate amounts of interallele recombination had also taken place. Surprisingly, the fliC locus contained a highly variable central region, even though insects lack an adaptive immune response, which is thought to drive flagellar variation in pathogens of higher organisms. All the X. nematophila strains exhibited a consistent pattern of insecticidal activity, and all contained the insecticidal toxin genes xptA1A2B1C1, which were present on a pathogenicity island (PAI). The PAIs were similar among the X. nematophila strains, except for partial deletions of a peptide synthetase gene and the presence of insertion sequences. Comparison of the PAI locus with that of X. bovienii suggested that the PAI integrated into the genome first and then acquired the xpt genes. The independent mobility of xpt genes was further supported by the presence of xpt genes in X. bovienii strain I73 on a type 2 transposon structure and by the variable patterns of insecticidal activity in X. bovienii isolates, even among closely related strains.
Figures




References
-
- Akhurst, R. J., and G. B. Dunphy. 1993. Symbiotically associated entomopathogenic bacteria, nematodes, and their insect hosts, p. 1-23. In S. T. N. Beckage and B. Federici (ed.), Parasites and pathogens of insects, vol. 2. Academic Press, New York, N.Y.
-
- Bellingham, N. F., J. A. W. Morgan, J. R. Saunders, and C. Winstanley. 2001. Flagellin gene sequence variation in the genus Pseudomonas. Syst. Appl. Microbiol. 24:157-165. - PubMed
-
- Bowen, D., T. A. Rocheleau, M. Blackburn, O. Andreev, E. Golubeva, R. Bhartia, and R. H. Ffrench-Constant. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280:2129-2132. - PubMed
-
- Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin, and F. Kunst. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 21:1307-1313. - PubMed
-
- Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

