Secretion of L-glutamate from osteoclasts through transcytosis
- PMID: 16957773
- PMCID: PMC1570443
- DOI: 10.1038/sj.emboj.7601317
Secretion of L-glutamate from osteoclasts through transcytosis
Abstract
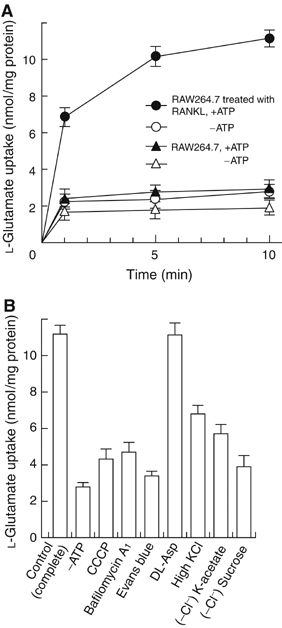
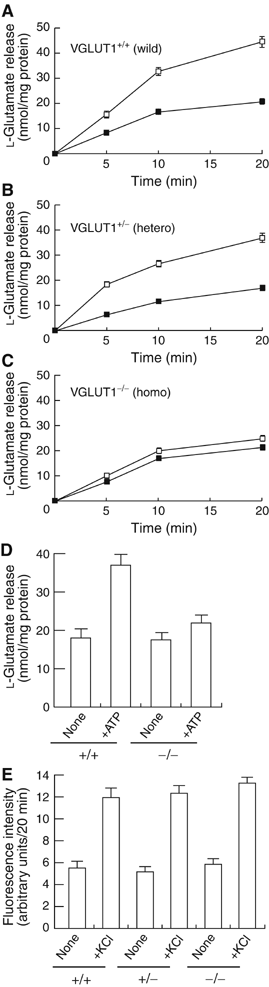
Osteoclasts are involved in the catabolism of the bone matrix and eliminate the resulting degradation products through transcytosis, but the molecular mechanism and regulation of transcytosis remain poorly understood. Upon differentiation, osteoclasts express vesicular glutamate transporter 1 (VGLUT1), which is essential for vesicular storage and subsequent exocytosis of glutamate in neurons. VGLUT1 is localized in transcytotic vesicles and accumulates L-glutamate. Osteoclasts secrete L-glutamate and the bone degradation products upon stimulation with KCl or ATP in a Ca2+-dependent manner. KCl- and ATP-dependent secretion of L-glutamate was absent in osteoclasts prepared from VGLUT1-/- knockout mice. Osteoclasts express mGluR8, a class III metabotropic glutamate receptor. Its stimulation by a specific agonist inhibits secretion of L-glutamate and bone degradation products, whereas its suppression by a specific antagonist stimulates bone resorption. Finally, it was found that VGLUT1-/- mice develop osteoporosis. Thus, in bone-resorbing osteoclasts, L-glutamate and bone degradation products are secreted through transcytosis and the released L-glutamate is involved in autoregulation of transcytosis. Glutamate signaling may play an important role in the bone homeostasis.
Figures






Similar articles
-
Vesicular storage and secretion of L-glutamate from glucagon-like peptide 1-secreting clonal intestinal L cells.J Neurochem. 2006 Jan;96(2):550-60. doi: 10.1111/j.1471-4159.2005.03575.x. Epub 2005 Dec 8. J Neurochem. 2006. PMID: 16336630
-
An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size.Proc Natl Acad Sci U S A. 2004 May 4;101(18):7158-63. doi: 10.1073/pnas.0401764101. Epub 2004 Apr 21. Proc Natl Acad Sci U S A. 2004. PMID: 15103023 Free PMC article.
-
Astrocytic exocytosis vesicles and glutamate: a high-resolution immunofluorescence study.Glia. 2005 Jan 1;49(1):96-106. doi: 10.1002/glia.20094. Glia. 2005. PMID: 15390103
-
Intracellular membrane trafficking in bone resorbing osteoclasts.Microsc Res Tech. 2003 Aug 15;61(6):496-503. doi: 10.1002/jemt.10371. Microsc Res Tech. 2003. PMID: 12879417 Review.
-
The role of glutamate transporters in bone cell signalling.J Musculoskelet Neuronal Interact. 2004 Jun;4(2):128-31. J Musculoskelet Neuronal Interact. 2004. PMID: 15615110 Review.
Cited by
-
Rab13 is upregulated during osteoclast differentiation and associates with small vesicles revealing polarized distribution in resorbing cells.J Histochem Cytochem. 2012 Jul;60(7):537-49. doi: 10.1369/0022155412448069. Epub 2012 May 4. J Histochem Cytochem. 2012. PMID: 22562557 Free PMC article.
-
Purinergic signalling in the musculoskeletal system.Purinergic Signal. 2013 Dec;9(4):541-72. doi: 10.1007/s11302-013-9381-4. Epub 2013 Aug 14. Purinergic Signal. 2013. PMID: 23943493 Free PMC article. Review.
-
Expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in the rat dental pulp and trigeminal ganglion following inflammation.PLoS One. 2014 Oct 7;9(10):e109723. doi: 10.1371/journal.pone.0109723. eCollection 2014. PLoS One. 2014. PMID: 25290694 Free PMC article.
-
SLC17A6/7/8 Vesicular Glutamate Transporter Homologs in Nematodes.Genetics. 2020 Jan;214(1):163-178. doi: 10.1534/genetics.119.302855. Epub 2019 Nov 27. Genetics. 2020. PMID: 31776169 Free PMC article.
-
Association Study between the FTCDNL1 (FONG) and Susceptibility to Osteoporosis.PLoS One. 2015 Oct 22;10(10):e0140549. doi: 10.1371/journal.pone.0140549. eCollection 2015. PLoS One. 2015. PMID: 26492493 Free PMC article.
References
-
- Acher FC, Tellier FJ, Azerad R, Brabet IN, Fagni L, Pin JP (1997) Synthesis and pharmacological characterization of aminocyclopentanetricarboxylic acids: new tools to discriminate between metabotropic glutamate receptor subtypes. J Med Chem 40: 3119–3129 - PubMed
-
- Baron R, Bartkiewicz M, David P, Hernando-Sobrino N (1994) Acidification and bone resorption: the role and characteristics of V-ATPases in the osteoclast. In Molecular Biology Intelligence Unit. Organellar Proton-ATPases, Nelson N (ed) pp 49–73. Springer-Verlag: RG Landes Company
-
- Bhangu PS, Genever PG, Spencer GJ, Grewal TS, Skerry TM (2001) Evidence for targeted vesicular glutamate exocytosis in osteoblasts. Bone 29: 16–23 - PubMed
-
- Blair HC (1998) How the osteoclast degrades bone. BioEssays 20: 837–846 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

