Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance
- PMID: 16957779
- PMCID: PMC1570444
- DOI: 10.1038/sj.emboj.7601303
Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance
Abstract
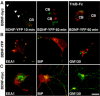
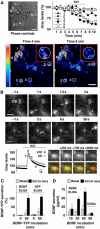
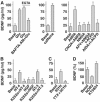
Regulation of brain-derived neurotrophic factor (BDNF) secretion plays a critical role in long-term potentiation (LTP). It is generally thought that the supply for this secretion is newly synthesized BDNF targeted to the synapse. Here we provide evidence that hippocampal neurons additionally recycle BDNF for activity-dependent secretion. Exogenously applied BDNF is internalized by cultured neurons and rapidly becomes available for activity-dependent secretion, which is controlled by the same mechanisms that regulate the secretion of newly synthesized BDNF. Moreover, BDNF recycling replaced the new synthesis pathway in mediating the maintenance of LTP in hippocampal slices: the late phase LTP, which is abolished by protein synthesis inhibition, was rescued in slices preincubated with BDNF. Thus, endocytosed BDNF is fed back to the activity-dependent releasable pool required for LTP maintenance.
Figures



References
-
- Ashby MC, Ibaraki K, Hemley JM (2004) It's green outside: tracking cell surface proteins with pH-sensitive GFP. Trends Neurosci 27: 257–261 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

